Sodium »
PDB 9dpp-9ewc »
9dpx »
Sodium in PDB 9dpx: Bmp-9 G389S K357R Dimer in Acidic pH
Protein crystallography data
The structure of Bmp-9 G389S K357R Dimer in Acidic pH, PDB code: 9dpx
was solved by
T.A.Schwartze,
A.P.Hinck,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 64.09 / 2.10 |
| Space group | I 41 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 71.289, 71.289, 146.379, 90, 90, 90 |
| R / Rfree (%) | 22.8 / 26.8 |
Other elements in 9dpx:
The structure of Bmp-9 G389S K357R Dimer in Acidic pH also contains other interesting chemical elements:
| Chlorine | (Cl) | 6 atoms |
Sodium Binding Sites:
The binding sites of Sodium atom in the Bmp-9 G389S K357R Dimer in Acidic pH
(pdb code 9dpx). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total 3 binding sites of Sodium where determined in the Bmp-9 G389S K357R Dimer in Acidic pH, PDB code: 9dpx:
Jump to Sodium binding site number: 1; 2; 3;
In total 3 binding sites of Sodium where determined in the Bmp-9 G389S K357R Dimer in Acidic pH, PDB code: 9dpx:
Jump to Sodium binding site number: 1; 2; 3;
Sodium binding site 1 out of 3 in 9dpx
Go back to
Sodium binding site 1 out
of 3 in the Bmp-9 G389S K357R Dimer in Acidic pH
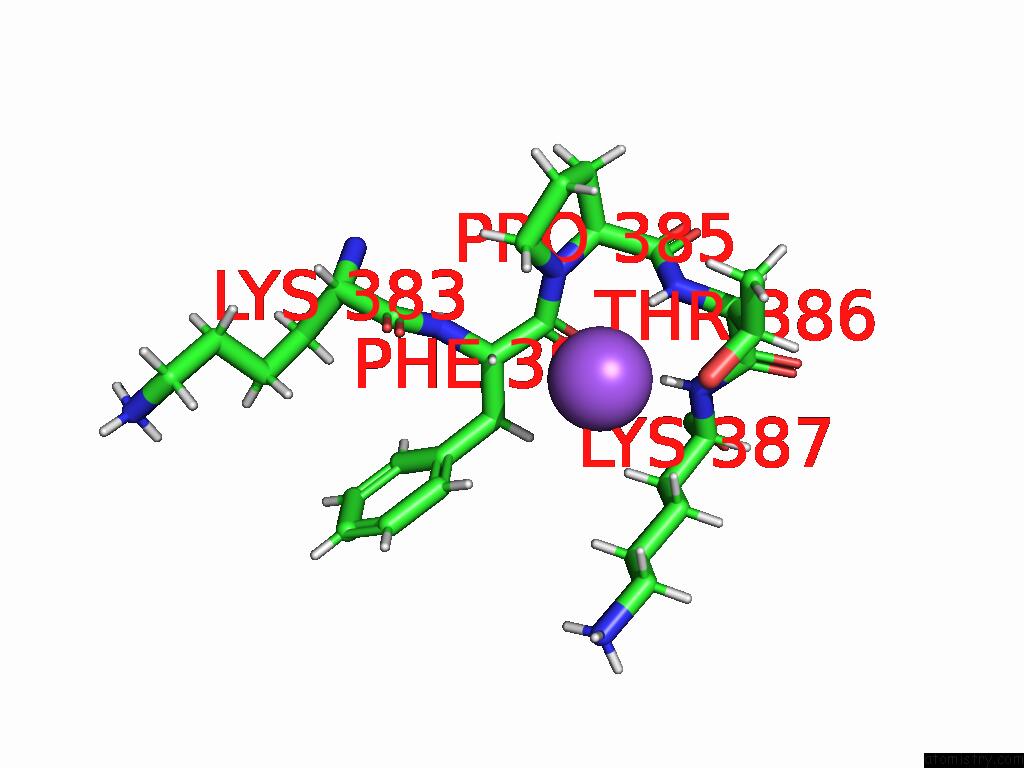
Mono view
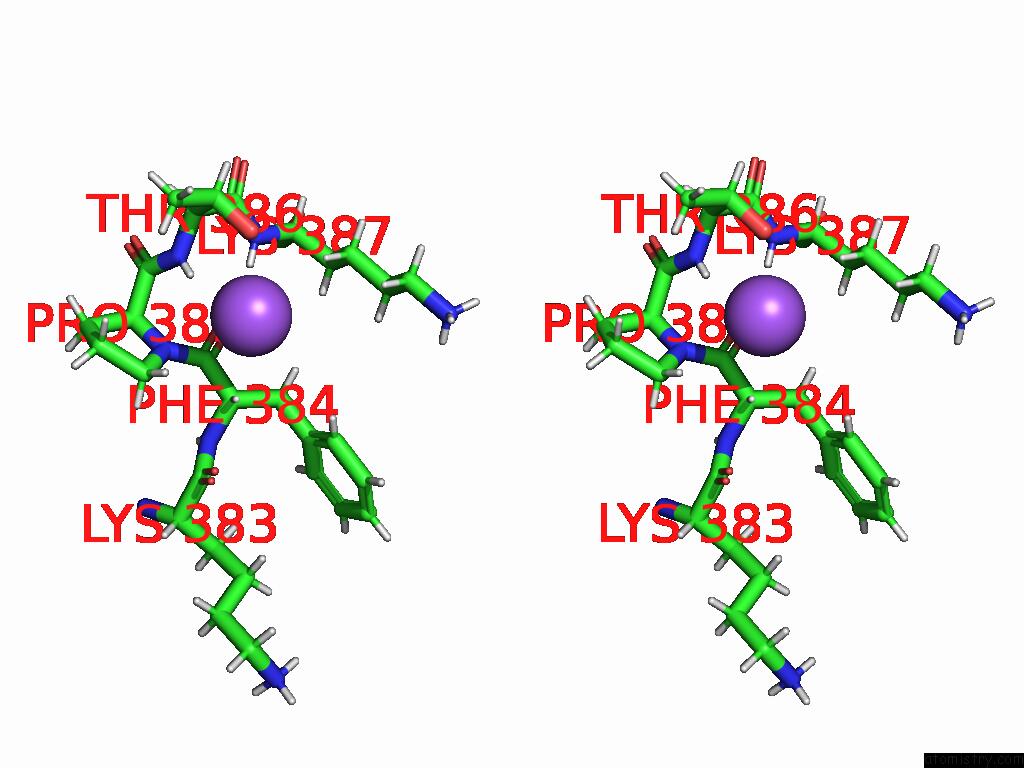
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Bmp-9 G389S K357R Dimer in Acidic pH within 5.0Å range:
|
Sodium binding site 2 out of 3 in 9dpx
Go back to
Sodium binding site 2 out
of 3 in the Bmp-9 G389S K357R Dimer in Acidic pH
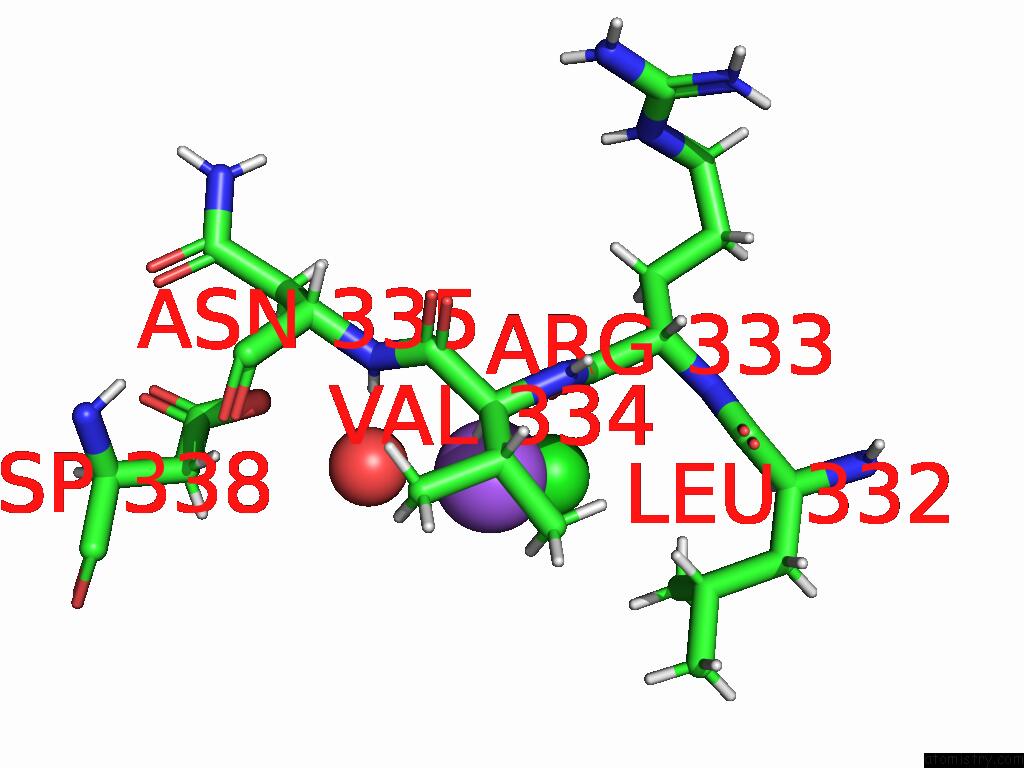
Mono view
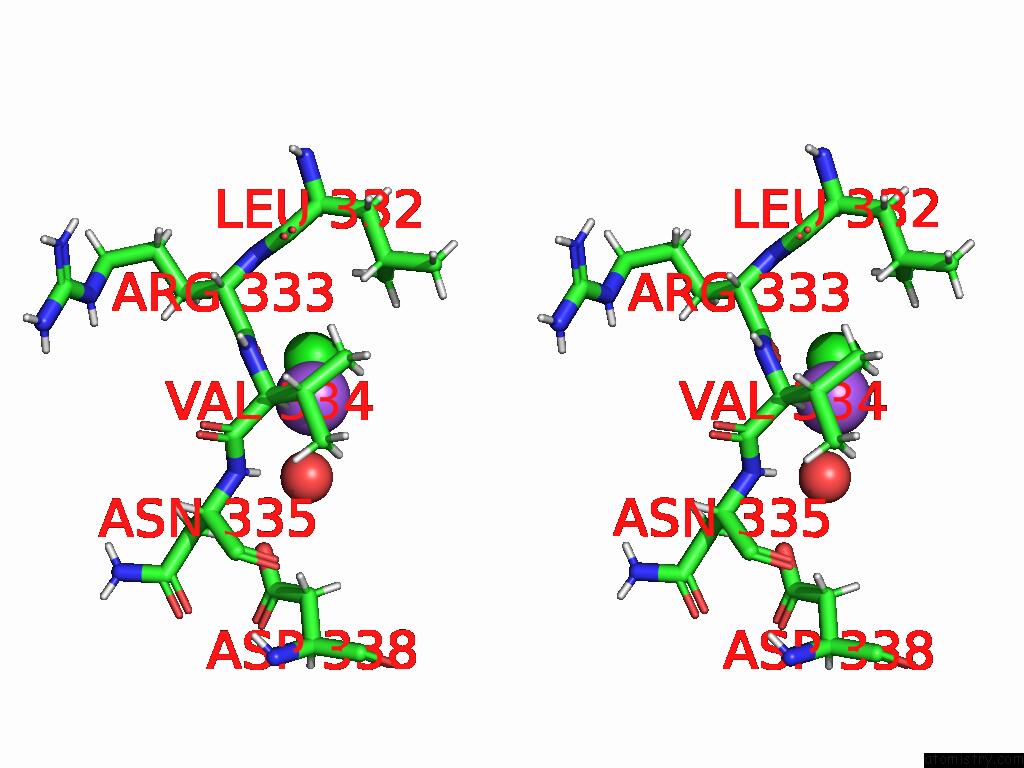
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 2 of Bmp-9 G389S K357R Dimer in Acidic pH within 5.0Å range:
|
Sodium binding site 3 out of 3 in 9dpx
Go back to
Sodium binding site 3 out
of 3 in the Bmp-9 G389S K357R Dimer in Acidic pH
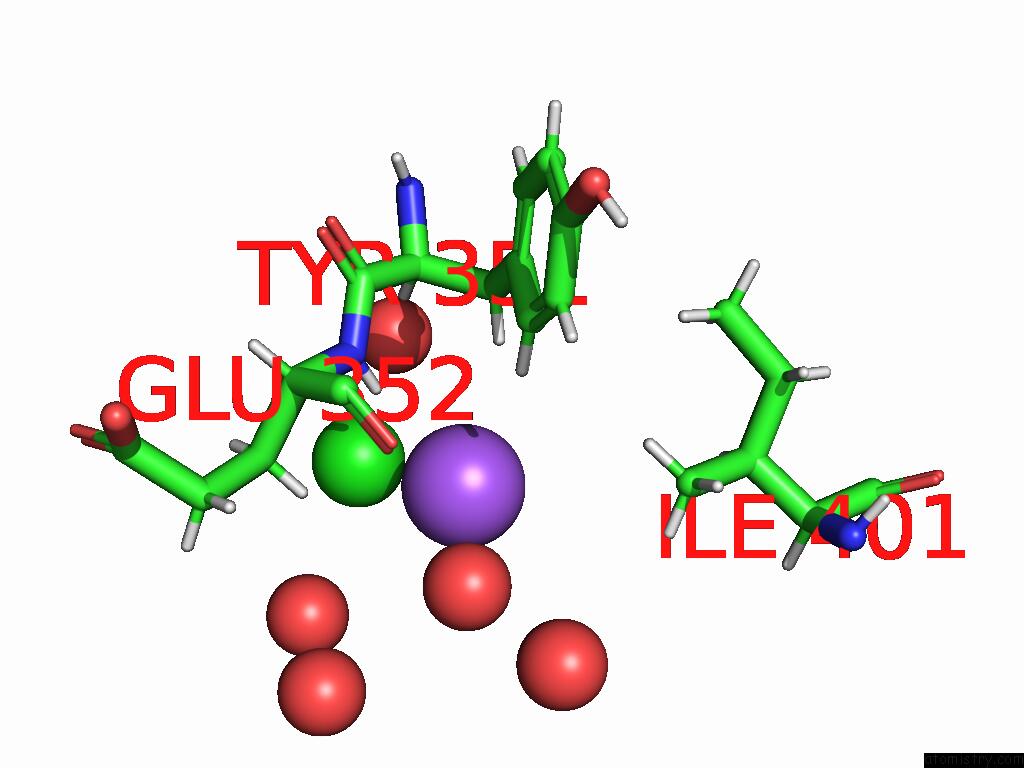
Mono view
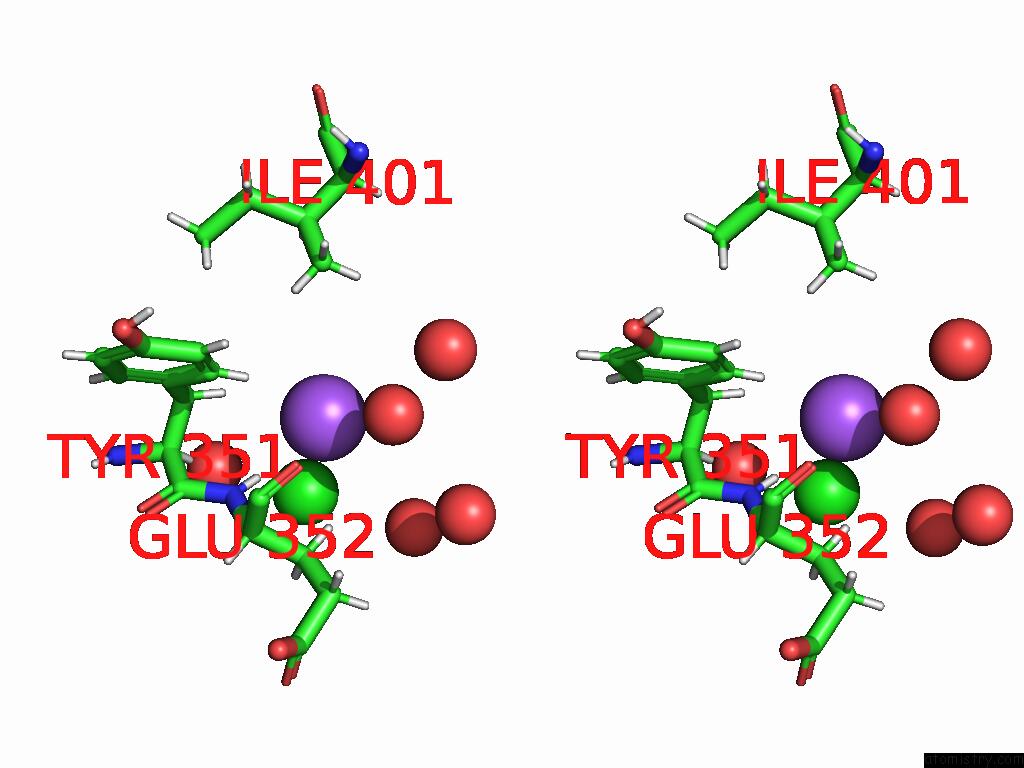
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 3 of Bmp-9 G389S K357R Dimer in Acidic pH within 5.0Å range:
|
Reference:
T.A.Schwartze,
S.A.Morosky,
T.L.Rosato,
A.Henrickson,
G.Lin,
C.S.Hinck,
A.B.Taylor,
S.K.Olsen,
G.Calero,
B.Demeler,
B.L.Roman,
A.P.Hinck.
Molecular Basis of Interchain Disulfide Bond Formation in Bmp-9 and Bmp-10. J.Mol.Biol. V. 437 68935 2025.
ISSN: ESSN 1089-8638
PubMed: 39793884
DOI: 10.1016/J.JMB.2025.168935
Page generated: Mon Aug 18 16:46:07 2025
ISSN: ESSN 1089-8638
PubMed: 39793884
DOI: 10.1016/J.JMB.2025.168935
Last articles
Mn in 9LJUMn in 9LJW
Mn in 9LJS
Mn in 9LJR
Mn in 9LJT
Mn in 9LJV
Mg in 9UA2
Mg in 9R96
Mg in 9VM1
Mg in 9P01