Sodium »
PDB 8vnr-8wch »
8w4x »
Sodium in PDB 8w4x: Neutron Structure of Cellulase CEL6A From Phanerochaete Chrysosporium at Room Temperature, Enzyme-Product Complex
Protein crystallography data
The structure of Neutron Structure of Cellulase CEL6A From Phanerochaete Chrysosporium at Room Temperature, Enzyme-Product Complex, PDB code: 8w4x
was solved by
M.Tachioka,
S.Yamaguchi,
A.Nakamura,
T.Ishida,
K.Kusaka,
T.Yamada,
N.Yano,
T.Chatake,
T.Tamada,
K.Takeda,
S.Niwa,
H.Tanaka,
S.Takahashi,
K.Inaka,
N.Furubayashi,
S.Deguchi,
M.Samejima,
K.Igarashi,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | N/A / 1.40 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 54.73, 68.26, 89.77, 90, 90, 90 |
| R / Rfree (%) | 14.7 / 16.6 |
Sodium Binding Sites:
The binding sites of Sodium atom in the Neutron Structure of Cellulase CEL6A From Phanerochaete Chrysosporium at Room Temperature, Enzyme-Product Complex
(pdb code 8w4x). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total only one binding site of Sodium was determined in the Neutron Structure of Cellulase CEL6A From Phanerochaete Chrysosporium at Room Temperature, Enzyme-Product Complex, PDB code: 8w4x:
In total only one binding site of Sodium was determined in the Neutron Structure of Cellulase CEL6A From Phanerochaete Chrysosporium at Room Temperature, Enzyme-Product Complex, PDB code: 8w4x:
Sodium binding site 1 out of 1 in 8w4x
Go back to
Sodium binding site 1 out
of 1 in the Neutron Structure of Cellulase CEL6A From Phanerochaete Chrysosporium at Room Temperature, Enzyme-Product Complex
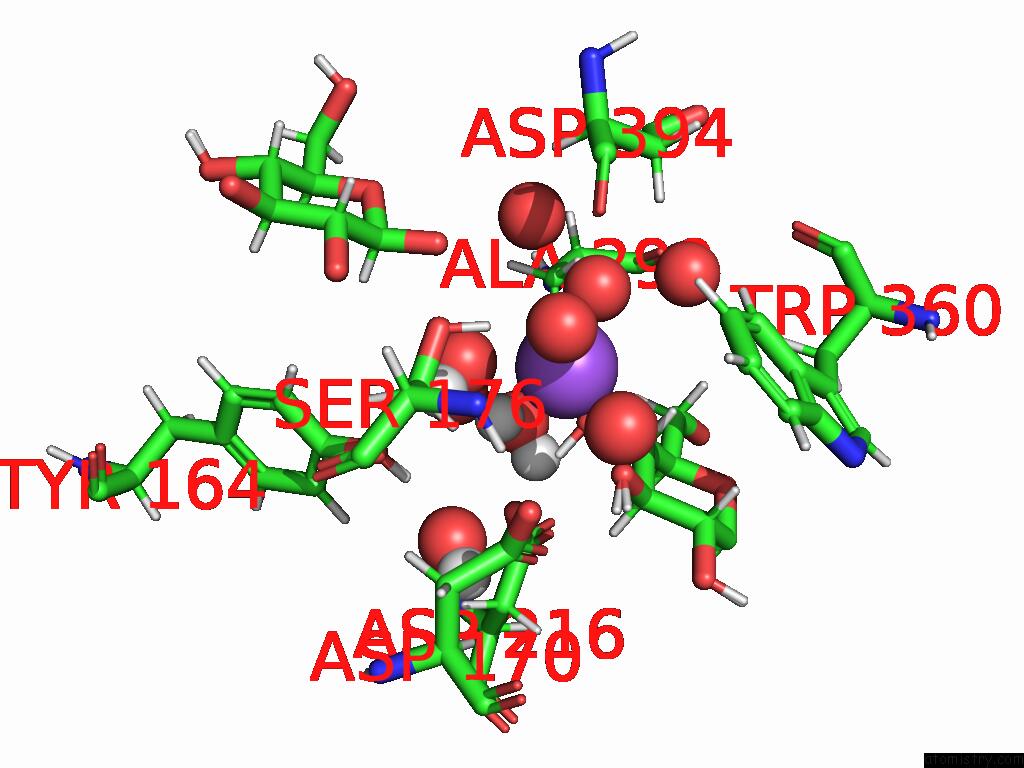
Mono view
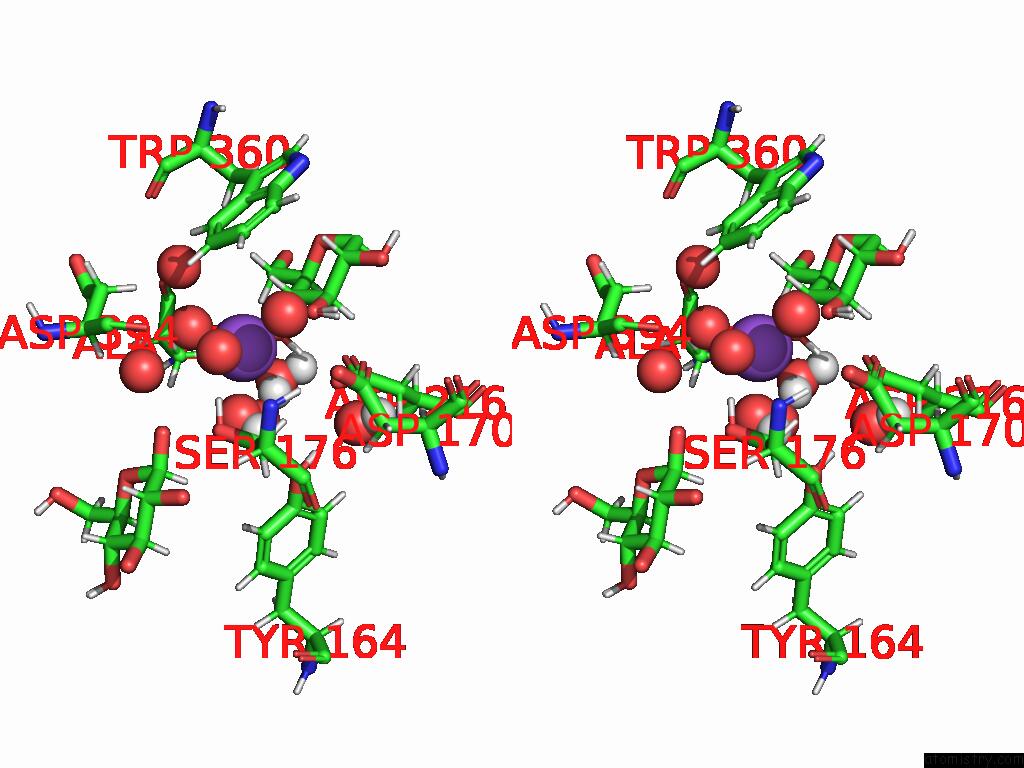
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Neutron Structure of Cellulase CEL6A From Phanerochaete Chrysosporium at Room Temperature, Enzyme-Product Complex within 5.0Å range:
|
Reference:
M.Tachioka,
S.Yamaguchi,
A.Nakamura,
T.Ishida,
K.Kusaka,
T.Yamada,
N.Yano,
T.Chatake,
T.Tamada,
K.Takeda,
S.Niwa,
H.Tanaka,
S.Takahashi,
K.Inaka,
N.Furubayashi,
S.Deguchi,
M.Samejima,
K.Igarashi.
Deprotonated Arginine Controls A Putative Catalytic Base in Invert-Ing Family 6 Glycoside Hydrolase To Be Published.
Page generated: Mon Aug 18 16:15:33 2025
Last articles
Ni in 7L1INi in 7JW4
Ni in 7L19
Ni in 7JM5
Ni in 7JYW
Ni in 7JVS
Ni in 7JGQ
Ni in 7JGP
Ni in 7JGO
Ni in 7JGK