Sodium »
PDB 7apy-7b7n »
7b3r »
Sodium in PDB 7b3r: Oxa-10 Beta-Lactamase with S64DHA Modification and Lysinoalanine Crosslink
Enzymatic activity of Oxa-10 Beta-Lactamase with S64DHA Modification and Lysinoalanine Crosslink
All present enzymatic activity of Oxa-10 Beta-Lactamase with S64DHA Modification and Lysinoalanine Crosslink:
3.5.2.6;
3.5.2.6;
Protein crystallography data
The structure of Oxa-10 Beta-Lactamase with S64DHA Modification and Lysinoalanine Crosslink, PDB code: 7b3r
was solved by
P.A.Lang,
J.Brem,
C.J.Schofield,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 63.09 / 1.83 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 48.61, 95.457, 126.186, 90, 90, 90 |
| R / Rfree (%) | 17 / 19.1 |
Sodium Binding Sites:
The binding sites of Sodium atom in the Oxa-10 Beta-Lactamase with S64DHA Modification and Lysinoalanine Crosslink
(pdb code 7b3r). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total only one binding site of Sodium was determined in the Oxa-10 Beta-Lactamase with S64DHA Modification and Lysinoalanine Crosslink, PDB code: 7b3r:
In total only one binding site of Sodium was determined in the Oxa-10 Beta-Lactamase with S64DHA Modification and Lysinoalanine Crosslink, PDB code: 7b3r:
Sodium binding site 1 out of 1 in 7b3r
Go back to
Sodium binding site 1 out
of 1 in the Oxa-10 Beta-Lactamase with S64DHA Modification and Lysinoalanine Crosslink
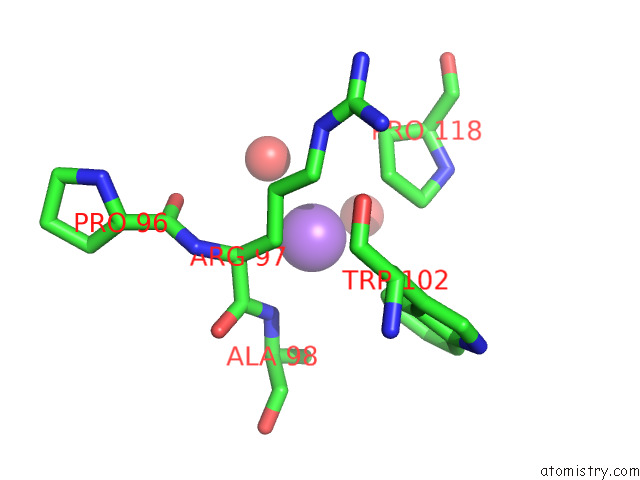
Mono view
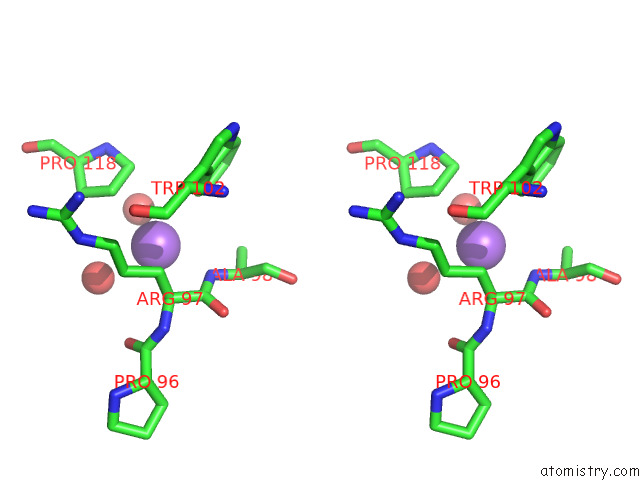
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Oxa-10 Beta-Lactamase with S64DHA Modification and Lysinoalanine Crosslink within 5.0Å range:
|
Reference:
P.A.Lang,
R.Raj,
A.Tumber,
C.T.Lohans,
P.Rabe,
C.V.Robinson,
J.Brem,
C.J.Schofield.
Studies on Enmetazobactam Clarify Mechanisms of Widely Used Beta-Lactamase Inhibitors. Proc.Natl.Acad.Sci.Usa V. 119 10119 2022.
ISSN: ESSN 1091-6490
PubMed: 35486701
DOI: 10.1073/PNAS.2117310119
Page generated: Mon Aug 18 09:15:01 2025
ISSN: ESSN 1091-6490
PubMed: 35486701
DOI: 10.1073/PNAS.2117310119
Last articles
Zn in 3N61Zn in 3N60
Zn in 3N5X
Zn in 3N5W
Zn in 3N5Z
Zn in 3N5Y
Zn in 3N5V
Zn in 3N5T
Zn in 3N5S
Zn in 3N5R