Sodium »
PDB 6nib-6nxy »
6nsv »
Sodium in PDB 6nsv: Crystal Structure of the Human Chip Tpr Domain in Complex with A 5MER Acetylated Optimized Peptide
Enzymatic activity of Crystal Structure of the Human Chip Tpr Domain in Complex with A 5MER Acetylated Optimized Peptide
All present enzymatic activity of Crystal Structure of the Human Chip Tpr Domain in Complex with A 5MER Acetylated Optimized Peptide:
2.3.2.27;
2.3.2.27;
Protein crystallography data
The structure of Crystal Structure of the Human Chip Tpr Domain in Complex with A 5MER Acetylated Optimized Peptide, PDB code: 6nsv
was solved by
K.Basu,
M.Ravalin,
M.-F.Bohn,
C.S.Craik,
J.E.Gestwicki,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 39.74 / 1.31 |
| Space group | P 2 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 46.219, 71.920, 77.790, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.4 / 24.3 |
Other elements in 6nsv:
The structure of Crystal Structure of the Human Chip Tpr Domain in Complex with A 5MER Acetylated Optimized Peptide also contains other interesting chemical elements:
| Chlorine | (Cl) | 1 atom |
Sodium Binding Sites:
The binding sites of Sodium atom in the Crystal Structure of the Human Chip Tpr Domain in Complex with A 5MER Acetylated Optimized Peptide
(pdb code 6nsv). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total 2 binding sites of Sodium where determined in the Crystal Structure of the Human Chip Tpr Domain in Complex with A 5MER Acetylated Optimized Peptide, PDB code: 6nsv:
Jump to Sodium binding site number: 1; 2;
In total 2 binding sites of Sodium where determined in the Crystal Structure of the Human Chip Tpr Domain in Complex with A 5MER Acetylated Optimized Peptide, PDB code: 6nsv:
Jump to Sodium binding site number: 1; 2;
Sodium binding site 1 out of 2 in 6nsv
Go back to
Sodium binding site 1 out
of 2 in the Crystal Structure of the Human Chip Tpr Domain in Complex with A 5MER Acetylated Optimized Peptide

Mono view
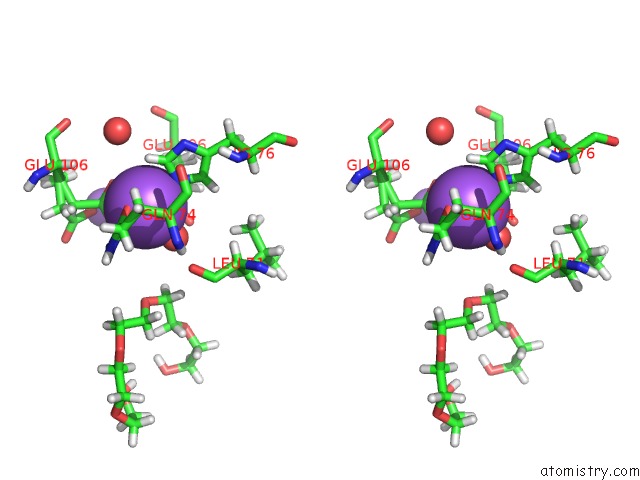
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Crystal Structure of the Human Chip Tpr Domain in Complex with A 5MER Acetylated Optimized Peptide within 5.0Å range:
|
Sodium binding site 2 out of 2 in 6nsv
Go back to
Sodium binding site 2 out
of 2 in the Crystal Structure of the Human Chip Tpr Domain in Complex with A 5MER Acetylated Optimized Peptide
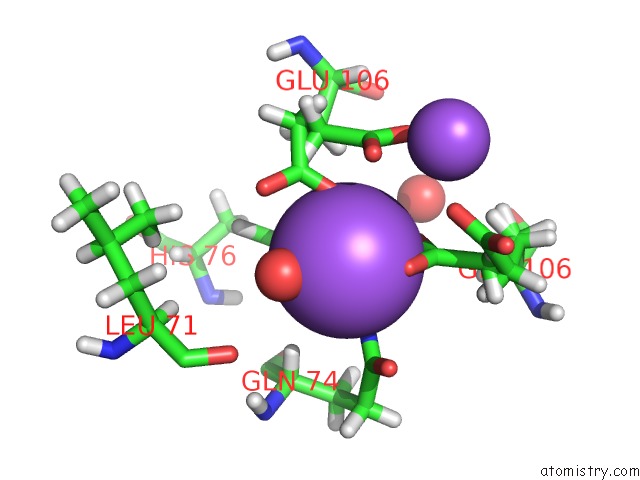
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 2 of Crystal Structure of the Human Chip Tpr Domain in Complex with A 5MER Acetylated Optimized Peptide within 5.0Å range:
|
Reference:
M.Ravalin,
P.Theofilas,
K.Basu,
K.A.Opoku-Nsiah,
V.A.Assimon,
D.Medina-Cleghorn,
Y.F.Chen,
M.F.Bohn,
M.Arkin,
L.T.Grinberg,
C.S.Craik,
J.E.Gestwicki.
Specificity For Latent C Termini Links the E3 Ubiquitin Ligase Chip to Caspases. Nat.Chem.Biol. V. 15 786 2019.
ISSN: ESSN 1552-4469
PubMed: 31320752
DOI: 10.1038/S41589-019-0322-6
Page generated: Tue Oct 8 12:21:38 2024
ISSN: ESSN 1552-4469
PubMed: 31320752
DOI: 10.1038/S41589-019-0322-6
Last articles
Cl in 5TPHCl in 5TPX
Cl in 5TPG
Cl in 5TOW
Cl in 5TNU
Cl in 5TPA
Cl in 5TOV
Cl in 5TP9
Cl in 5TOO
Cl in 5TO1