Sodium »
PDB 6dq0-6e8t »
6dwd »
Sodium in PDB 6dwd: SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket
Protein crystallography data
The structure of SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket, PDB code: 6dwd
was solved by
K.M.Knecht,
O.Buzovetsky,
C.Schneider,
D.Thomas,
V.Srikanth,
L.Kaderali,
F.Tofoleanu,
K.Reiss,
N.Ferreiros,
G.Geisslinger,
V.S.Batista,
X.Ji,
J.Cinatl,
O.T.Keppler,
Y.Xiong,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 1.70 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 80.477, 142.233, 98.790, 90.00, 114.10, 90.00 |
| R / Rfree (%) | 17.6 / 20.2 |
Other elements in 6dwd:
The structure of SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket also contains other interesting chemical elements:
| Fluorine | (F) | 8 atoms |
| Magnesium | (Mg) | 10 atoms |
Sodium Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 20; Page 3, Binding sites: 21 - 23;Binding sites:
The binding sites of Sodium atom in the SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket (pdb code 6dwd). This binding sites where shown within 5.0 Angstroms radius around Sodium atom.In total 23 binding sites of Sodium where determined in the SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket, PDB code: 6dwd:
Jump to Sodium binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Sodium binding site 1 out of 23 in 6dwd
Go back to
Sodium binding site 1 out
of 23 in the SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket
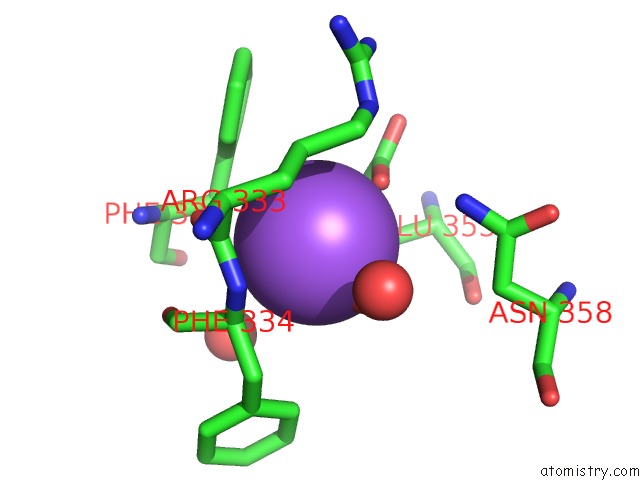
Mono view
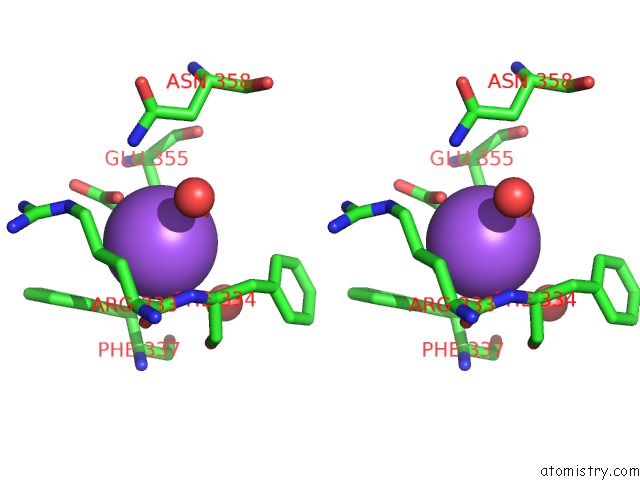
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket within 5.0Å range:
|
Sodium binding site 2 out of 23 in 6dwd
Go back to
Sodium binding site 2 out
of 23 in the SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket
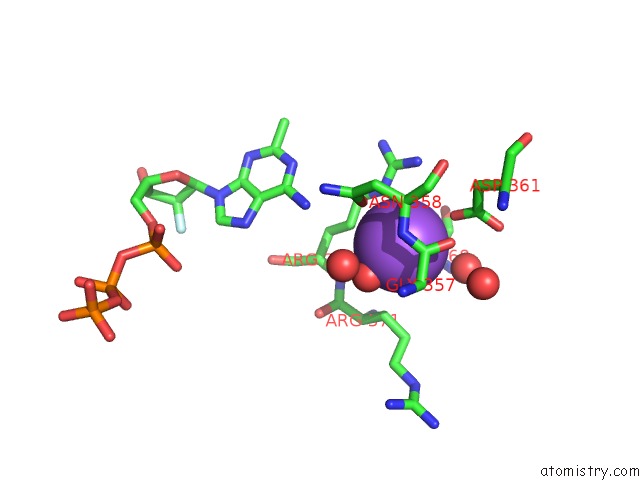
Mono view
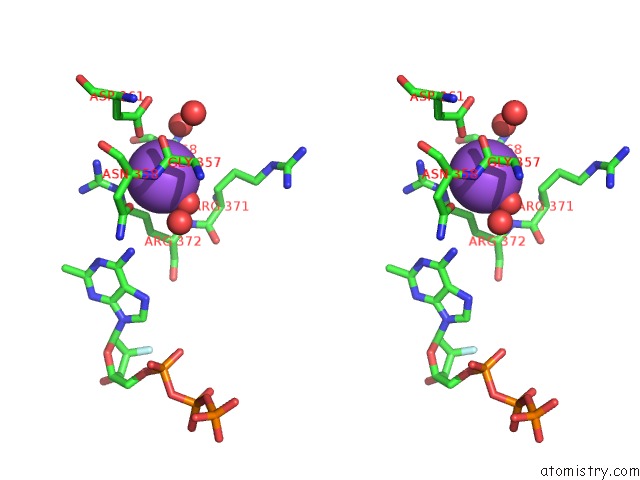
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 2 of SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket within 5.0Å range:
|
Sodium binding site 3 out of 23 in 6dwd
Go back to
Sodium binding site 3 out
of 23 in the SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket
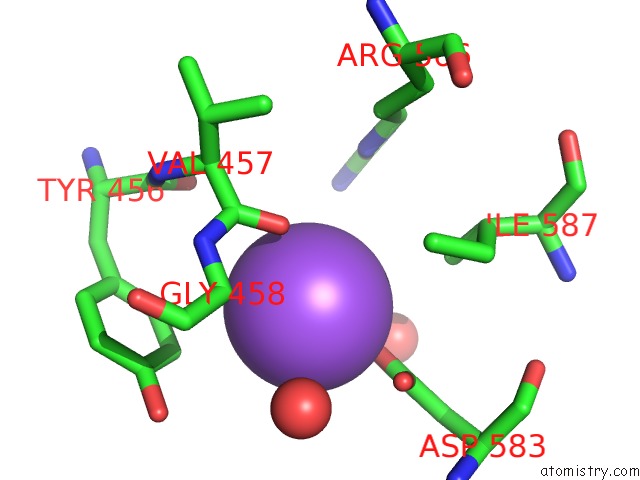
Mono view
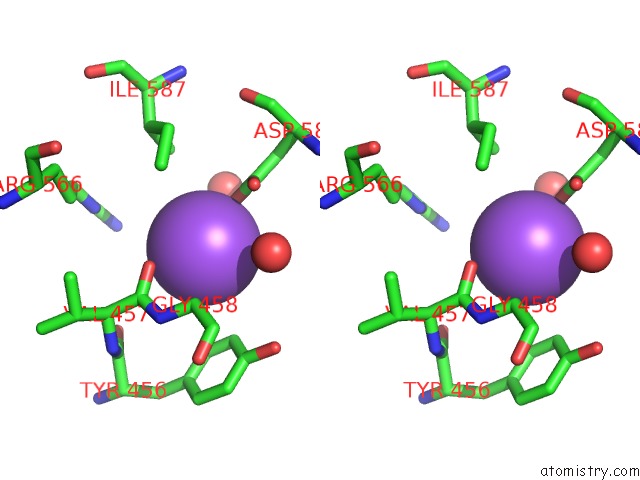
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 3 of SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket within 5.0Å range:
|
Sodium binding site 4 out of 23 in 6dwd
Go back to
Sodium binding site 4 out
of 23 in the SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket
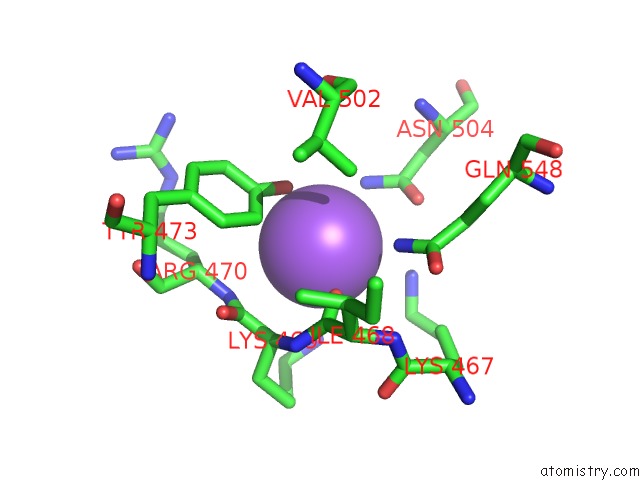
Mono view
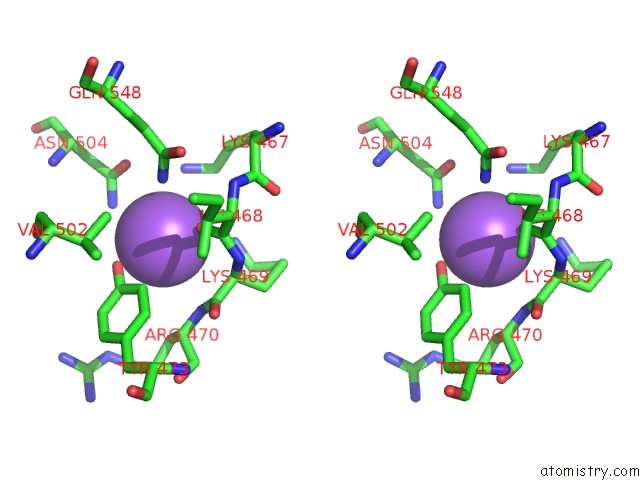
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 4 of SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket within 5.0Å range:
|
Sodium binding site 5 out of 23 in 6dwd
Go back to
Sodium binding site 5 out
of 23 in the SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket
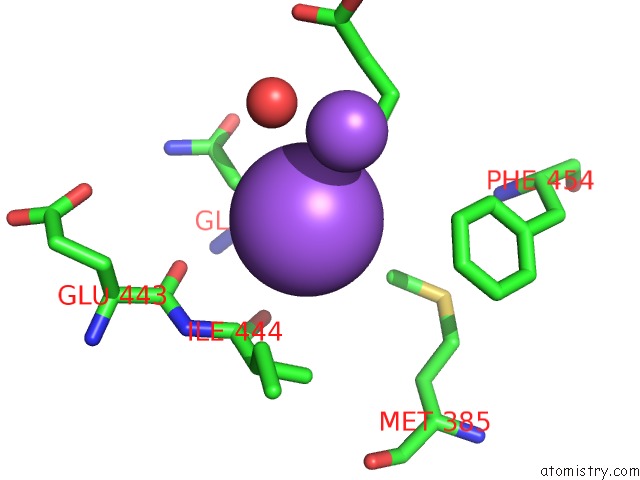
Mono view
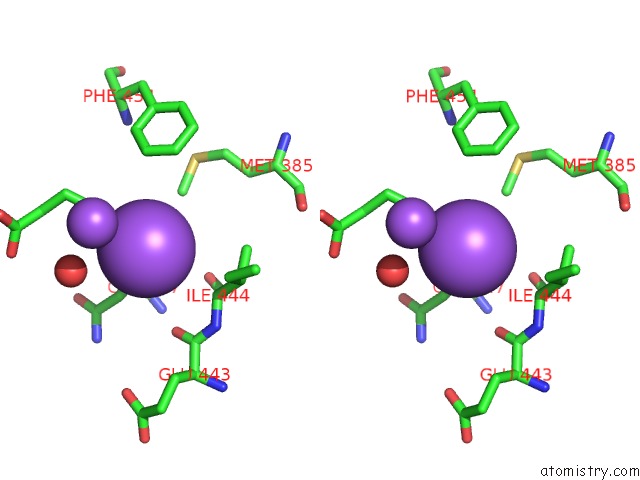
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 5 of SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket within 5.0Å range:
|
Sodium binding site 6 out of 23 in 6dwd
Go back to
Sodium binding site 6 out
of 23 in the SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket
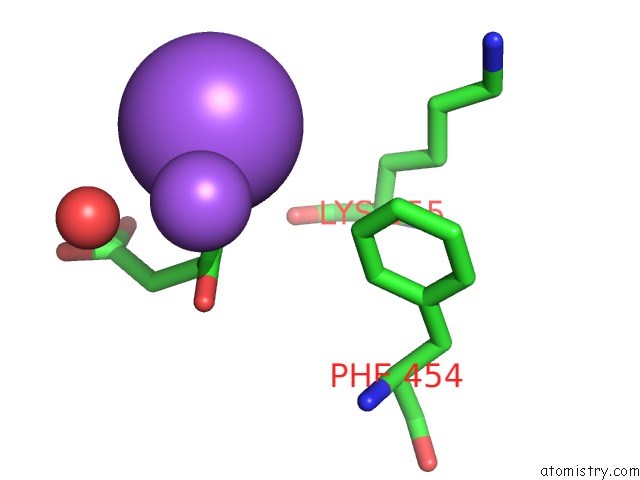
Mono view
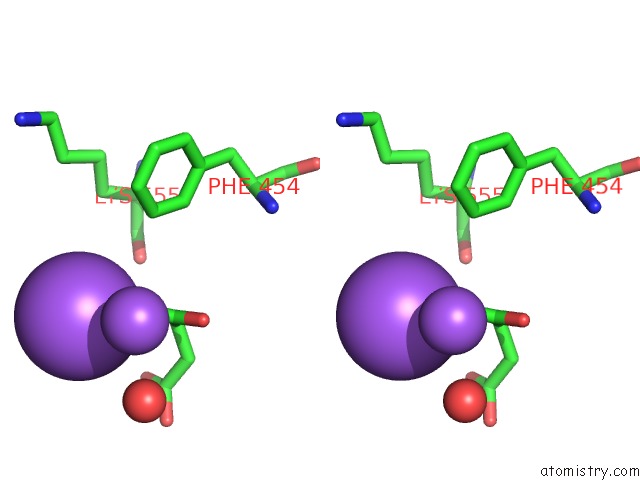
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 6 of SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket within 5.0Å range:
|
Sodium binding site 7 out of 23 in 6dwd
Go back to
Sodium binding site 7 out
of 23 in the SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket
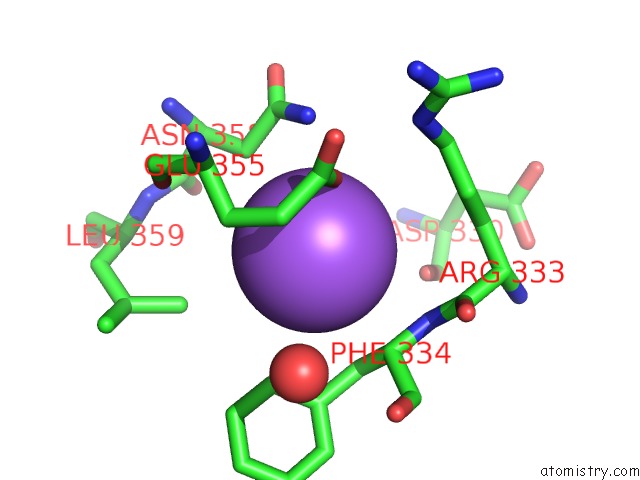
Mono view
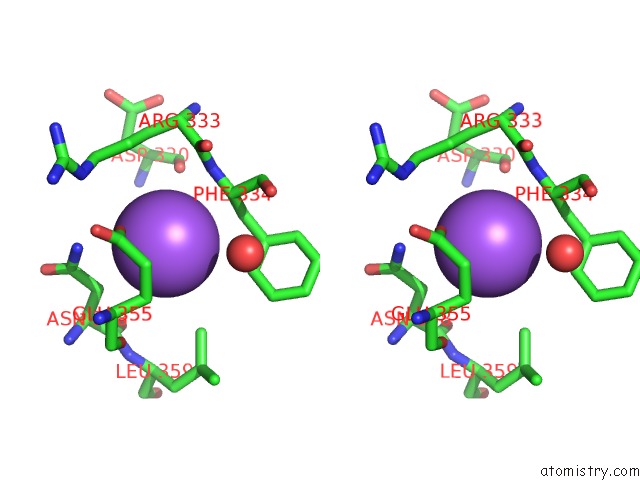
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 7 of SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket within 5.0Å range:
|
Sodium binding site 8 out of 23 in 6dwd
Go back to
Sodium binding site 8 out
of 23 in the SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket
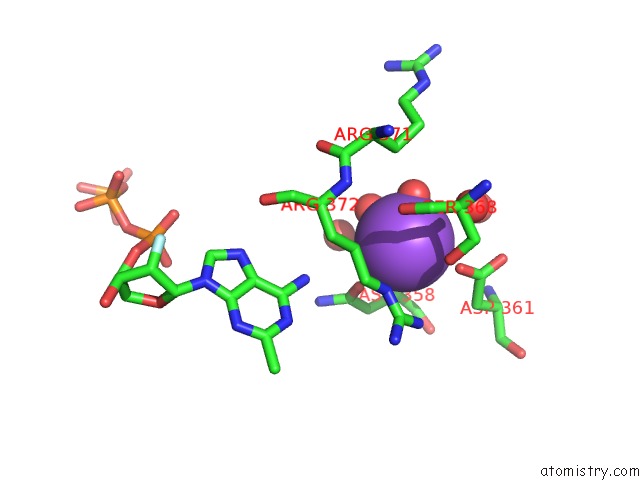
Mono view
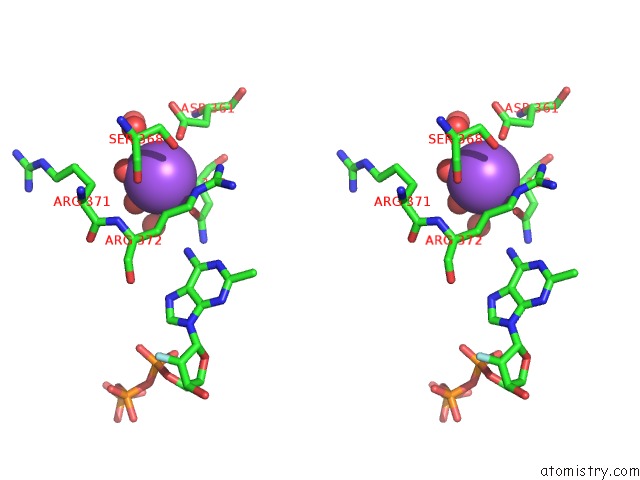
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 8 of SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket within 5.0Å range:
|
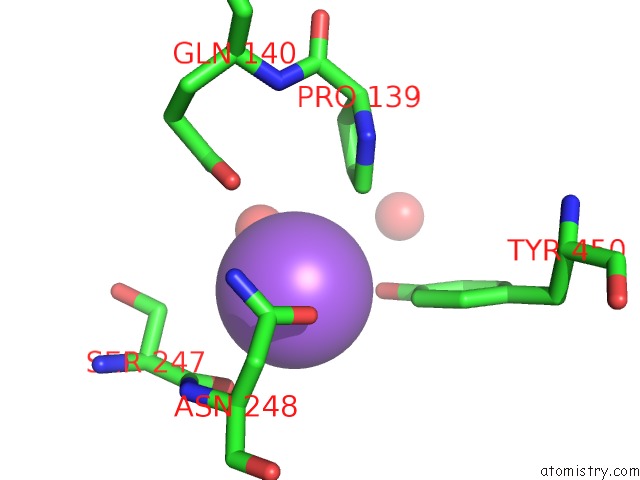
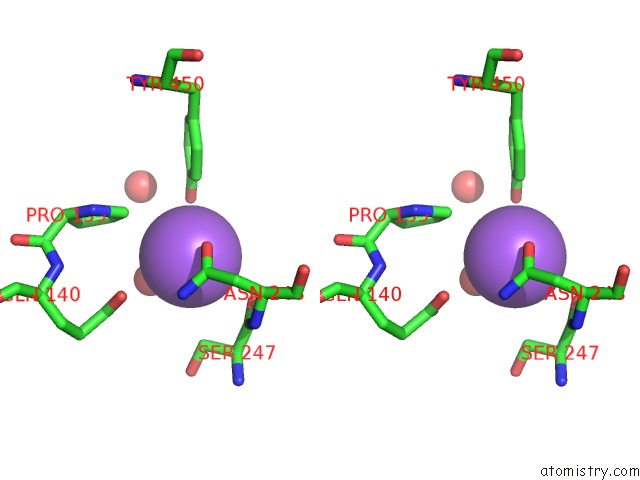
Sodium binding site 9 out of 23 in 6dwd
Go back to
Sodium binding site 9 out
of 23 in the SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 9 of SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket within 5.0Å range:
|
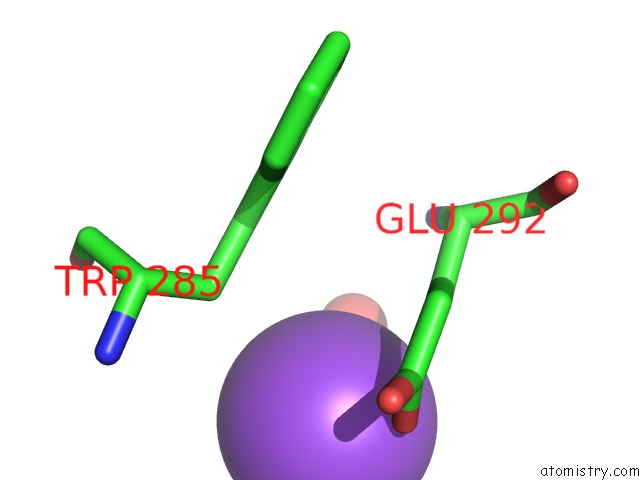
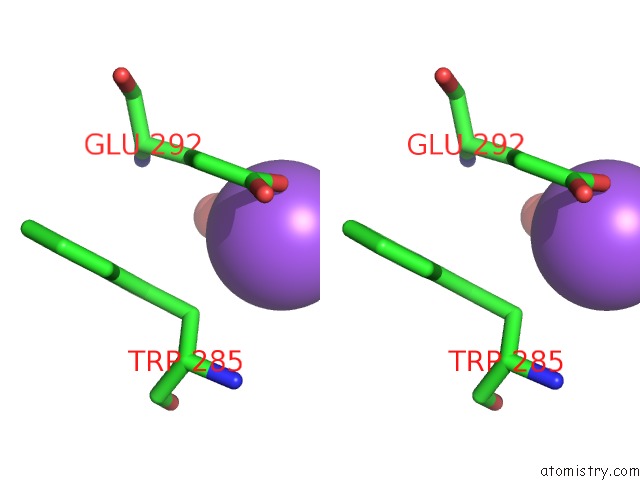
Sodium binding site 10 out of 23 in 6dwd
Go back to
Sodium binding site 10 out
of 23 in the SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 10 of SAMHD1 Bound to Clofarabine-Tp in the Catalytic Pocket and Allosteric Pocket within 5.0Å range:
|
Reference:
K.M.Knecht,
O.Buzovetsky,
C.Schneider,
D.Thomas,
V.Srikanth,
L.Kaderali,
F.Tofoleanu,
K.Reiss,
N.Ferreiros,
G.Geisslinger,
V.S.Batista,
X.Ji,
J.Cinatl Jr.,
O.T.Keppler,
Y.Xiong.
The Structural Basis For Cancer Drug Interactions with the Catalytic and Allosteric Sites of SAMHD1. Proc. Natl. Acad. Sci. V. 115 10022 2018U.S.A..
ISSN: ESSN 1091-6490
PubMed: 30305425
DOI: 10.1073/PNAS.1805593115
Page generated: Tue Oct 8 08:11:11 2024
ISSN: ESSN 1091-6490
PubMed: 30305425
DOI: 10.1073/PNAS.1805593115
Last articles
Cl in 5TR9Cl in 5TQU
Cl in 5TQJ
Cl in 5TPI
Cl in 5TQI
Cl in 5TPU
Cl in 5TPH
Cl in 5TPX
Cl in 5TPG
Cl in 5TOW