Sodium »
PDB 5o8n-5opd »
5oe2 »
Sodium in PDB 5oe2: Crystal Structure of the Beta-Lactamase Oxa-245
Enzymatic activity of Crystal Structure of the Beta-Lactamase Oxa-245
All present enzymatic activity of Crystal Structure of the Beta-Lactamase Oxa-245:
3.5.2.6;
3.5.2.6;
Protein crystallography data
The structure of Crystal Structure of the Beta-Lactamase Oxa-245, PDB code: 5oe2
was solved by
B.A.Lund,
H.K.S.Leiros,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 45.26 / 2.20 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 64.164, 108.720, 83.677, 90.00, 102.39, 90.00 |
| R / Rfree (%) | 19.1 / 22.6 |
Other elements in 5oe2:
The structure of Crystal Structure of the Beta-Lactamase Oxa-245 also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
Sodium Binding Sites:
The binding sites of Sodium atom in the Crystal Structure of the Beta-Lactamase Oxa-245
(pdb code 5oe2). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total only one binding site of Sodium was determined in the Crystal Structure of the Beta-Lactamase Oxa-245, PDB code: 5oe2:
In total only one binding site of Sodium was determined in the Crystal Structure of the Beta-Lactamase Oxa-245, PDB code: 5oe2:
Sodium binding site 1 out of 1 in 5oe2
Go back to
Sodium binding site 1 out
of 1 in the Crystal Structure of the Beta-Lactamase Oxa-245
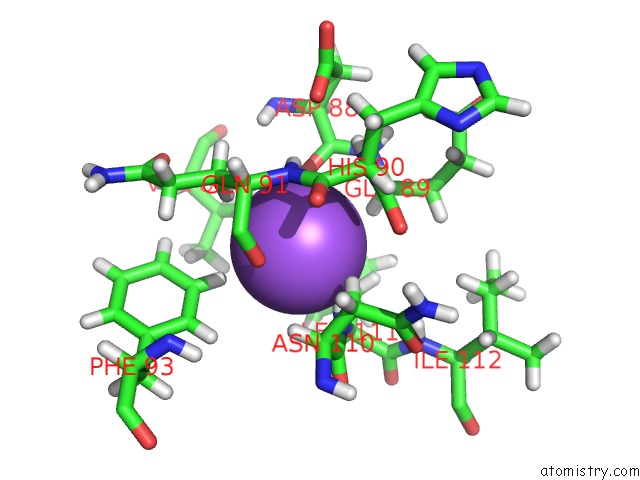
Mono view
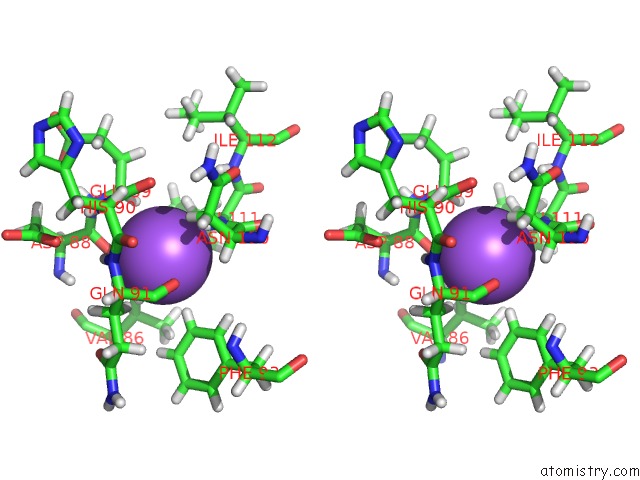
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Crystal Structure of the Beta-Lactamase Oxa-245 within 5.0Å range:
|
Reference:
B.A.Lund,
A.M.Thomassen,
T.J.O.Carlsen,
H.K.S.Leiros.
Structure, Activity and Thermostability Investigations of Oxa-163, Oxa-181 and Oxa-245 Using Biochemical Analysis, Crystal Structures and Differential Scanning Calorimetry Analysis. Acta Crystallogr F Struct V. 73 579 2017BIOL Commun.
ISSN: ESSN 2053-230X
PubMed: 28994407
DOI: 10.1107/S2053230X17013838
Page generated: Mon Aug 18 01:31:00 2025
ISSN: ESSN 2053-230X
PubMed: 28994407
DOI: 10.1107/S2053230X17013838
Last articles
Na in 6NYPNa in 6NXS
Na in 6NXX
Na in 6NXY
Na in 6NW9
Na in 6NXR
Na in 6NVA
Na in 6NXQ
Na in 6NVU
Na in 6NUF