Sodium »
PDB 5iaj-5imz »
5ibc »
Sodium in PDB 5ibc: Caspase 3 V266I
Enzymatic activity of Caspase 3 V266I
All present enzymatic activity of Caspase 3 V266I:
3.4.22.56;
3.4.22.56;
Protein crystallography data
The structure of Caspase 3 V266I, PDB code: 5ibc
was solved by
J.J.Maciag,
S.H.Mackenzie,
M.B.Tucker,
J.L.Schipper,
P.D.Swartz,
A.C.Clark,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 23.98 / 1.66 |
| Space group | I 2 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 68.591, 84.325, 96.617, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.2 / 20.8 |
Other elements in 5ibc:
The structure of Caspase 3 V266I also contains other interesting chemical elements:
| Chlorine | (Cl) | 1 atom |
Sodium Binding Sites:
The binding sites of Sodium atom in the Caspase 3 V266I
(pdb code 5ibc). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total only one binding site of Sodium was determined in the Caspase 3 V266I, PDB code: 5ibc:
In total only one binding site of Sodium was determined in the Caspase 3 V266I, PDB code: 5ibc:
Sodium binding site 1 out of 1 in 5ibc
Go back to
Sodium binding site 1 out
of 1 in the Caspase 3 V266I
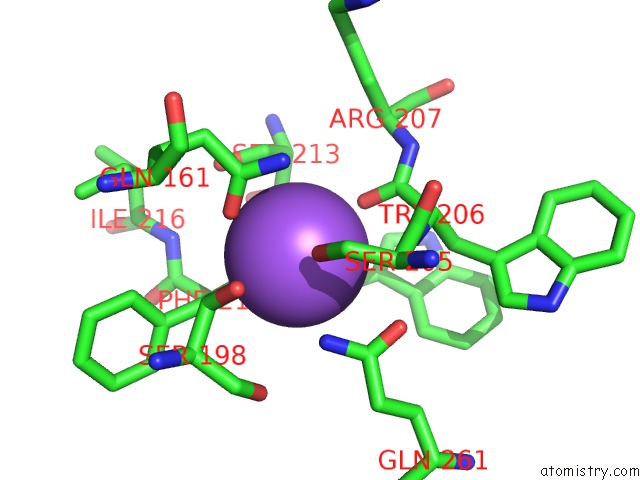
Mono view
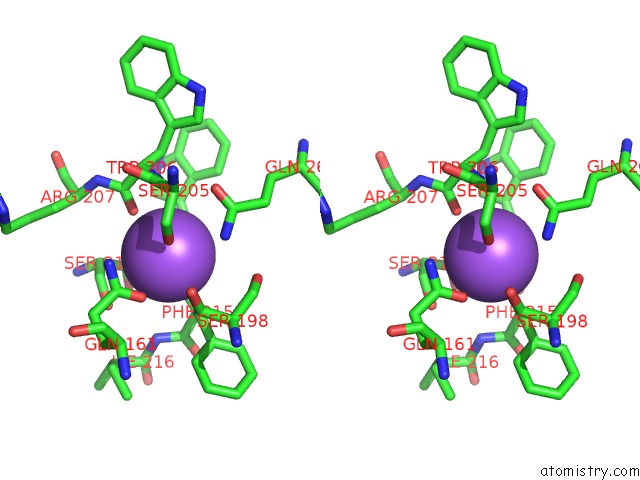
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Caspase 3 V266I within 5.0Å range:
|
Reference:
J.J.Maciag,
S.H.Mackenzie,
M.B.Tucker,
J.L.Schipper,
P.Swartz,
A.C.Clark.
Tunable Allosteric Library of Caspase-3 Identifies Coupling Between Conserved Water Molecules and Conformational Selection. Proc.Natl.Acad.Sci.Usa V. 113 E6080 2016.
ISSN: ESSN 1091-6490
PubMed: 27681633
DOI: 10.1073/PNAS.1603549113
Page generated: Mon Oct 7 21:34:08 2024
ISSN: ESSN 1091-6490
PubMed: 27681633
DOI: 10.1073/PNAS.1603549113
Last articles
Cl in 5EAECl in 5EAD
Cl in 5E9R
Cl in 5EAC
Cl in 5EAB
Cl in 5EA6
Cl in 5E9X
Cl in 5E8R
Cl in 5E9K
Cl in 5E9C