Sodium »
PDB 4khu-4kxx »
4kxx »
Sodium in PDB 4kxx: Human Transketolase in Covalent Complex with Donor Ketose D- Sedoheptulose-7-Phosphate
Enzymatic activity of Human Transketolase in Covalent Complex with Donor Ketose D- Sedoheptulose-7-Phosphate
All present enzymatic activity of Human Transketolase in Covalent Complex with Donor Ketose D- Sedoheptulose-7-Phosphate:
2.2.1.1;
2.2.1.1;
Protein crystallography data
The structure of Human Transketolase in Covalent Complex with Donor Ketose D- Sedoheptulose-7-Phosphate, PDB code: 4kxx
was solved by
P.Neumann,
S.Luedtke,
R.Ficner,
K.Tittmann,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 30.00 / 1.03 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 113.675, 86.097, 73.017, 90.00, 125.43, 90.00 |
| R / Rfree (%) | 11.7 / 14.8 |
Other elements in 4kxx:
The structure of Human Transketolase in Covalent Complex with Donor Ketose D- Sedoheptulose-7-Phosphate also contains other interesting chemical elements:
| Magnesium | (Mg) | 1 atom |
Sodium Binding Sites:
The binding sites of Sodium atom in the Human Transketolase in Covalent Complex with Donor Ketose D- Sedoheptulose-7-Phosphate
(pdb code 4kxx). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total only one binding site of Sodium was determined in the Human Transketolase in Covalent Complex with Donor Ketose D- Sedoheptulose-7-Phosphate, PDB code: 4kxx:
In total only one binding site of Sodium was determined in the Human Transketolase in Covalent Complex with Donor Ketose D- Sedoheptulose-7-Phosphate, PDB code: 4kxx:
Sodium binding site 1 out of 1 in 4kxx
Go back to
Sodium binding site 1 out
of 1 in the Human Transketolase in Covalent Complex with Donor Ketose D- Sedoheptulose-7-Phosphate
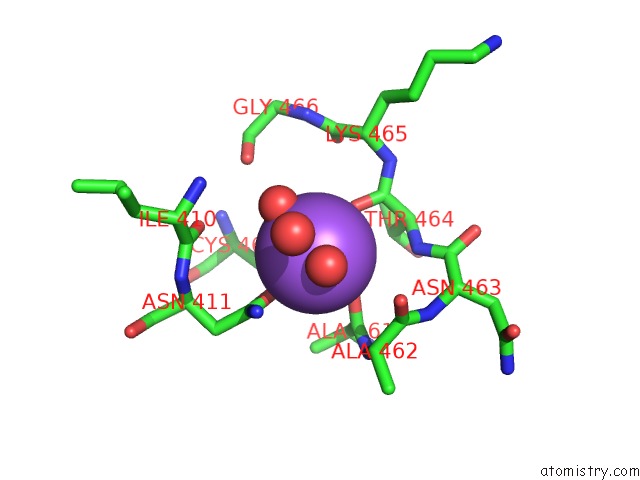
Mono view
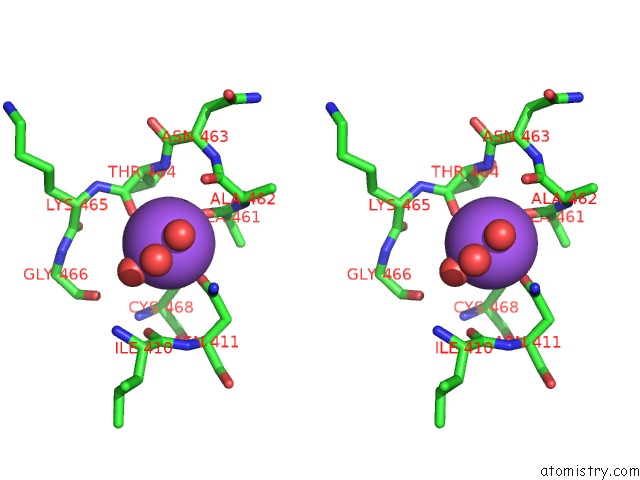
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Human Transketolase in Covalent Complex with Donor Ketose D- Sedoheptulose-7-Phosphate within 5.0Å range:
|
Reference:
S.Ludtke,
P.Neumann,
K.M.Erixon,
F.Leeper,
R.Kluger,
R.Ficner,
K.Tittmann.
Sub-Angstrom-Resolution Crystallography Reveals Physical Distortions That Enhance Reactivity of A Covalent Enzymatic Intermediate. Nat Chem V. 5 762 2013.
PubMed: 23965678
DOI: 10.1038/NCHEM.1728
Page generated: Sun Aug 17 20:20:36 2025
PubMed: 23965678
DOI: 10.1038/NCHEM.1728
Last articles
Na in 6CTNNa in 6CTF
Na in 6CTL
Na in 6CTM
Na in 6CTK
Na in 6CTI
Na in 6CTJ
Na in 6CSE
Na in 6CSF
Na in 6CRH