Sodium »
PDB 4c6s-4cnt »
4cdb »
Sodium in PDB 4cdb: Crystal Structure of Listeriolysin O
Protein crystallography data
The structure of Crystal Structure of Listeriolysin O, PDB code: 4cdb
was solved by
S.Koester,
O.Yildiz,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.638 / 2.15 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 26.720, 85.150, 229.900, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 19.37 / 24.59 |
Sodium Binding Sites:
Pages:
>>> Page 1 <<< Page 2, Binding sites: 11 - 15;Binding sites:
The binding sites of Sodium atom in the Crystal Structure of Listeriolysin O (pdb code 4cdb). This binding sites where shown within 5.0 Angstroms radius around Sodium atom.In total 15 binding sites of Sodium where determined in the Crystal Structure of Listeriolysin O, PDB code: 4cdb:
Jump to Sodium binding site number: 1; 2; 3; 4; 5; 6; 7; 8; 9; 10;
Sodium binding site 1 out of 15 in 4cdb
Go back to
Sodium binding site 1 out
of 15 in the Crystal Structure of Listeriolysin O
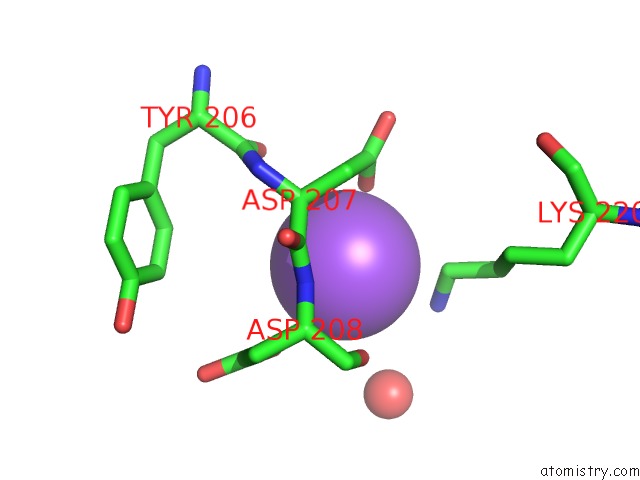
Mono view
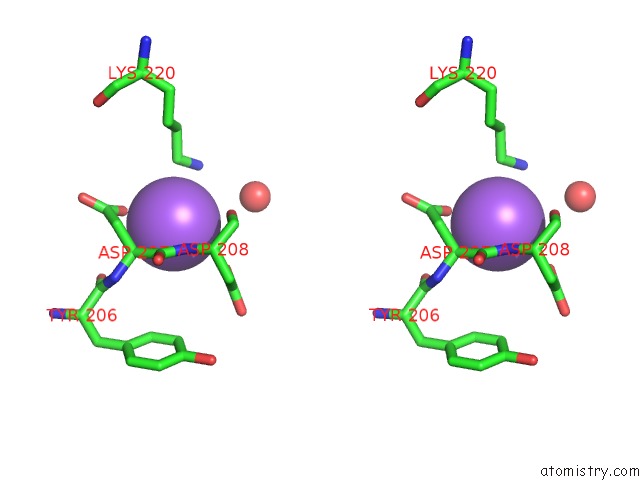
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Crystal Structure of Listeriolysin O within 5.0Å range:
|
Sodium binding site 2 out of 15 in 4cdb
Go back to
Sodium binding site 2 out
of 15 in the Crystal Structure of Listeriolysin O
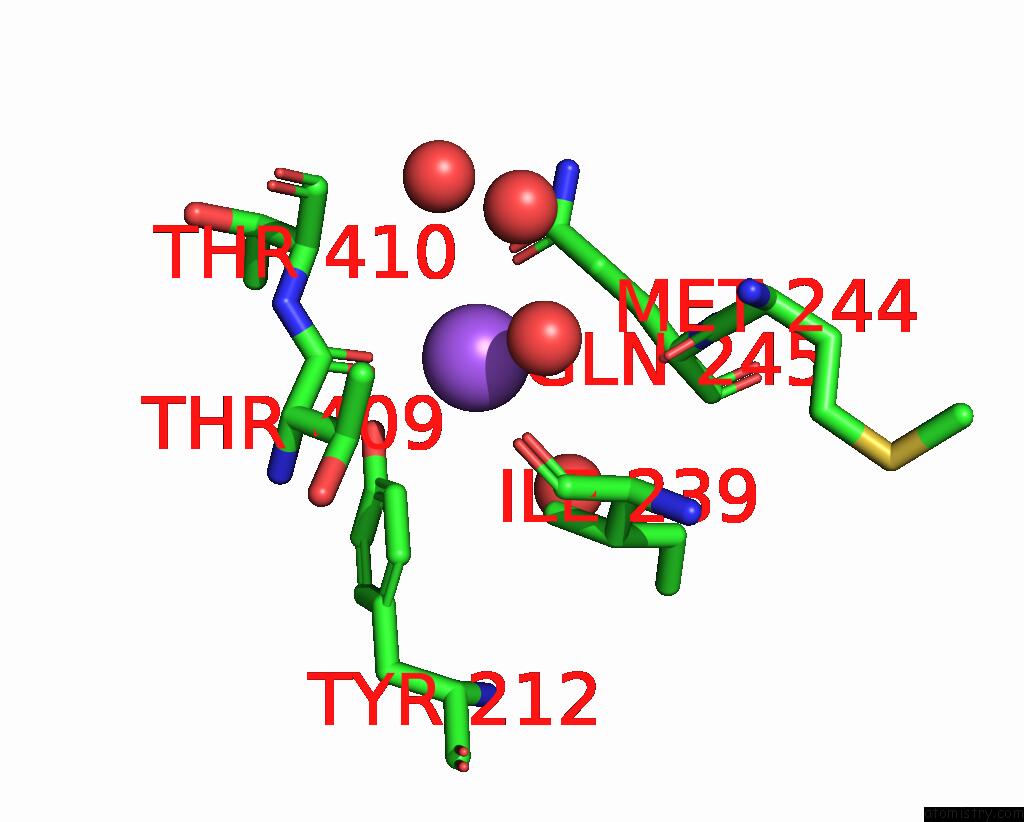
Mono view
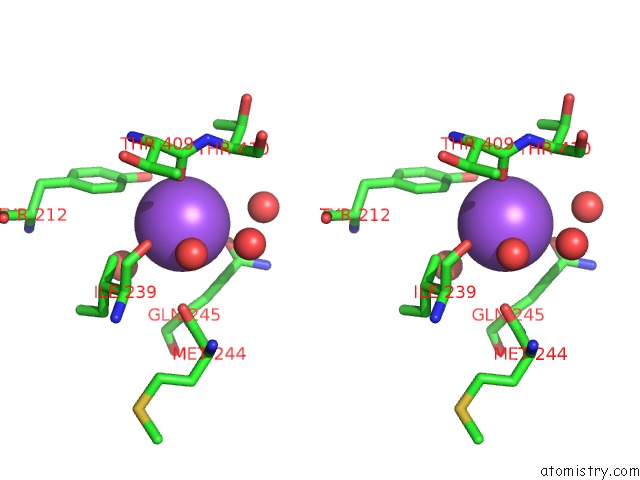
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 2 of Crystal Structure of Listeriolysin O within 5.0Å range:
|
Sodium binding site 3 out of 15 in 4cdb
Go back to
Sodium binding site 3 out
of 15 in the Crystal Structure of Listeriolysin O
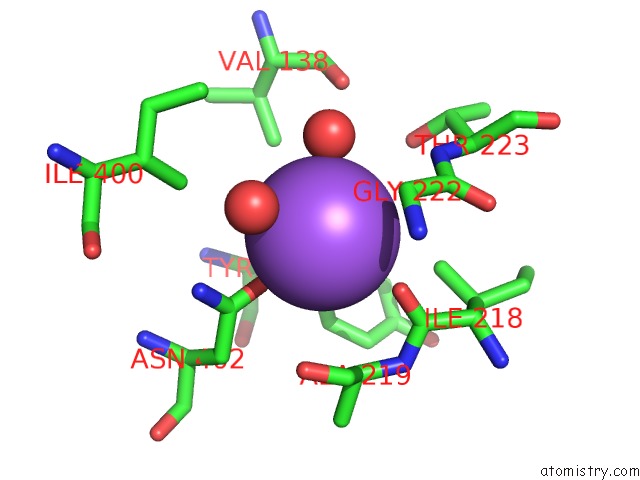
Mono view
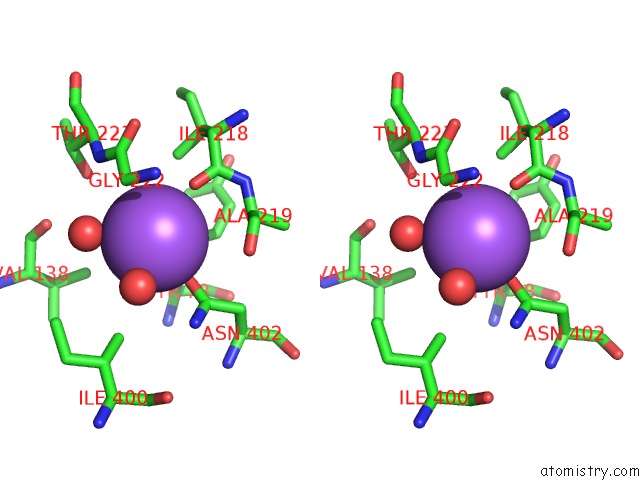
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 3 of Crystal Structure of Listeriolysin O within 5.0Å range:
|
Sodium binding site 4 out of 15 in 4cdb
Go back to
Sodium binding site 4 out
of 15 in the Crystal Structure of Listeriolysin O
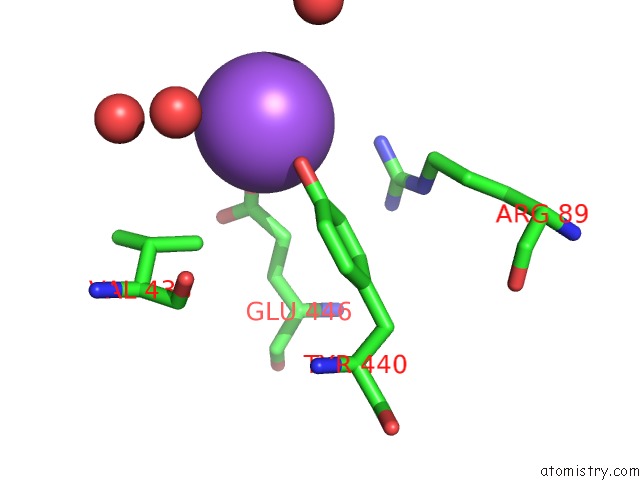
Mono view
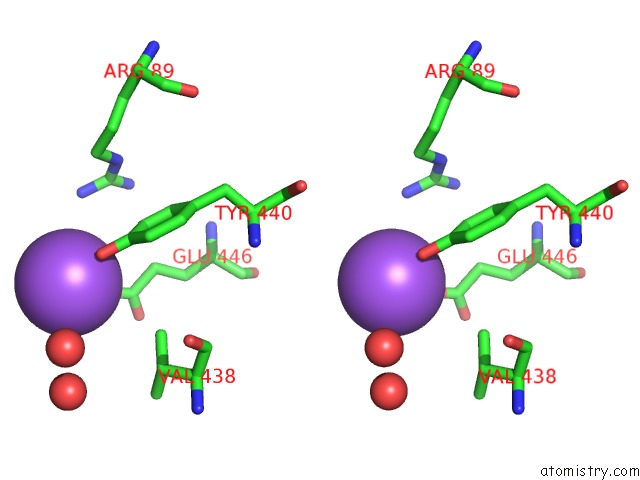
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 4 of Crystal Structure of Listeriolysin O within 5.0Å range:
|
Sodium binding site 5 out of 15 in 4cdb
Go back to
Sodium binding site 5 out
of 15 in the Crystal Structure of Listeriolysin O
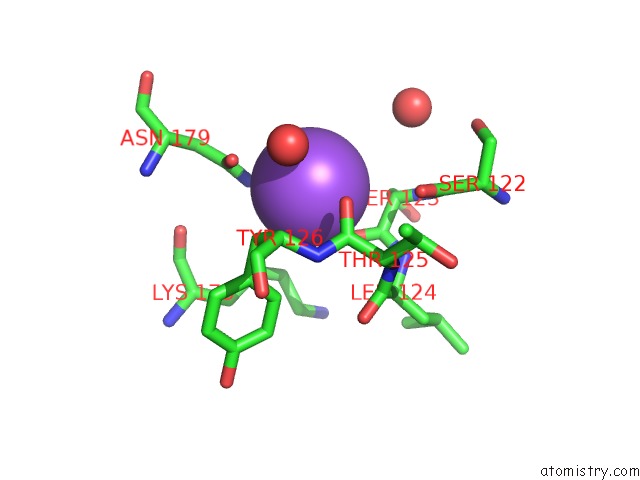
Mono view
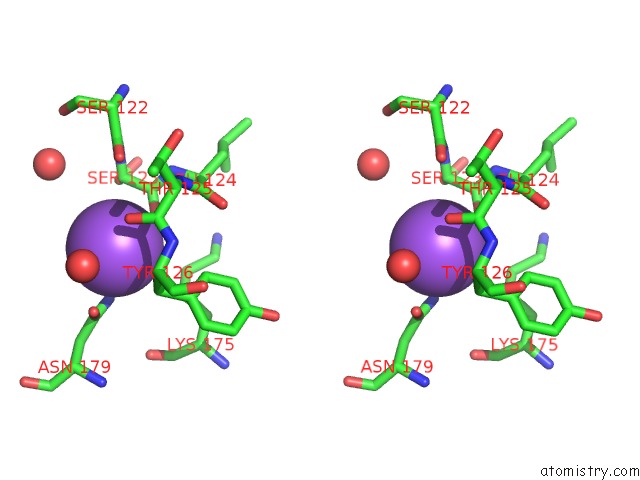
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 5 of Crystal Structure of Listeriolysin O within 5.0Å range:
|
Sodium binding site 6 out of 15 in 4cdb
Go back to
Sodium binding site 6 out
of 15 in the Crystal Structure of Listeriolysin O
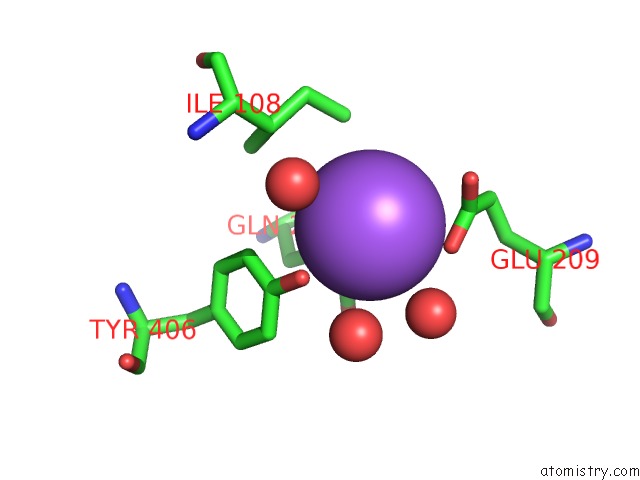
Mono view
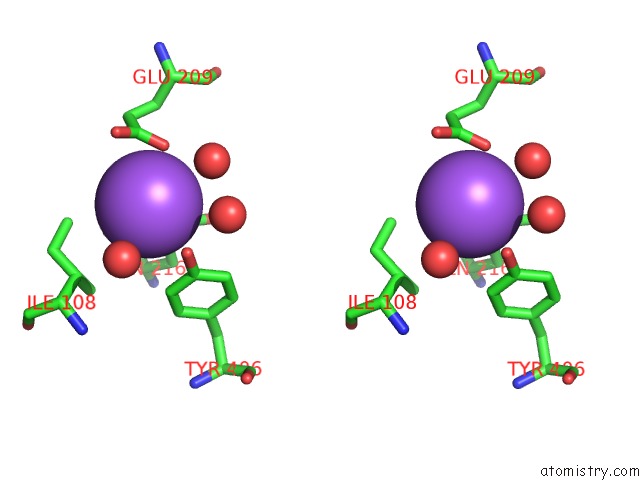
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 6 of Crystal Structure of Listeriolysin O within 5.0Å range:
|
Sodium binding site 7 out of 15 in 4cdb
Go back to
Sodium binding site 7 out
of 15 in the Crystal Structure of Listeriolysin O
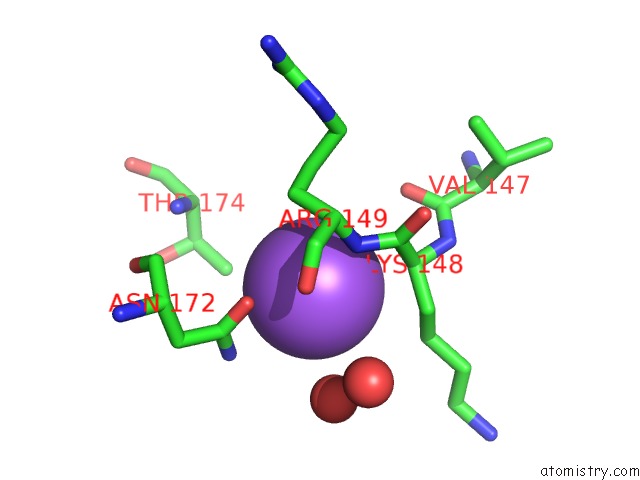
Mono view
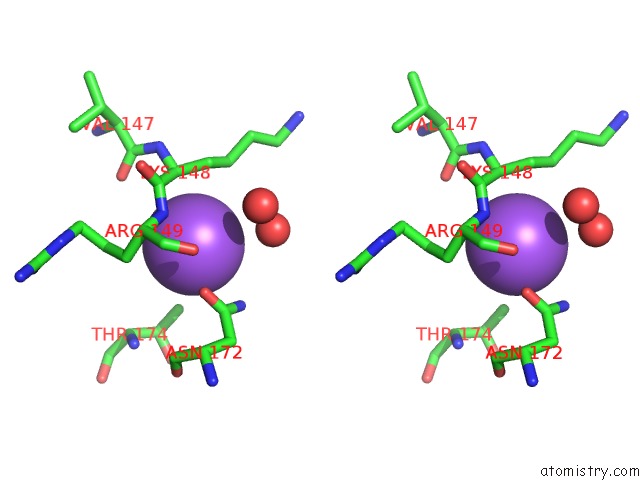
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 7 of Crystal Structure of Listeriolysin O within 5.0Å range:
|
Sodium binding site 8 out of 15 in 4cdb
Go back to
Sodium binding site 8 out
of 15 in the Crystal Structure of Listeriolysin O
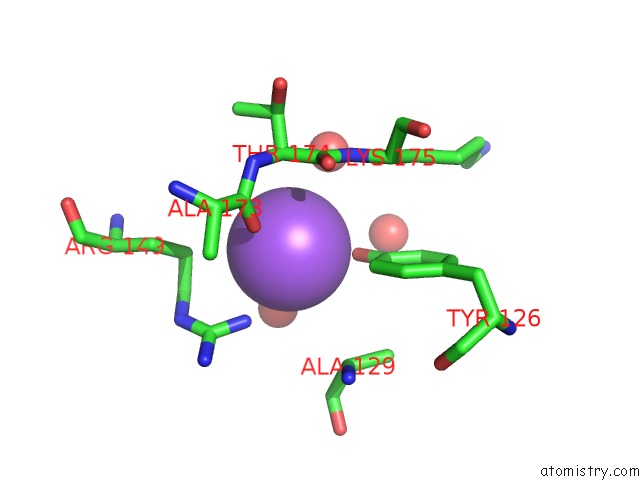
Mono view
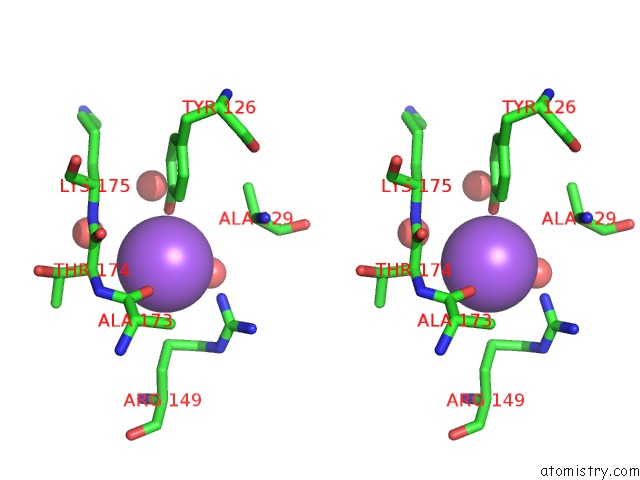
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 8 of Crystal Structure of Listeriolysin O within 5.0Å range:
|
Sodium binding site 9 out of 15 in 4cdb
Go back to
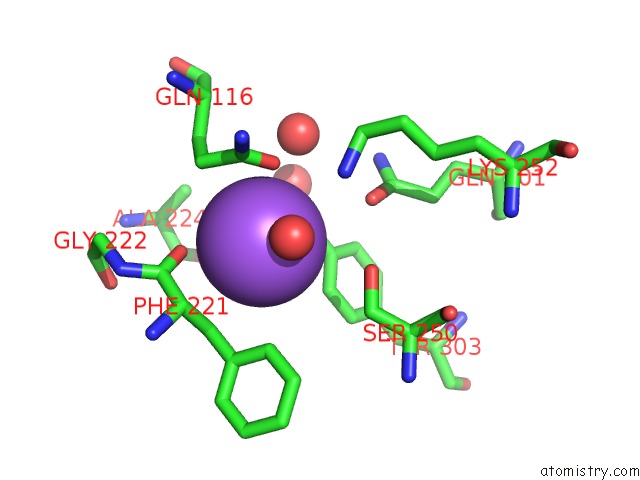
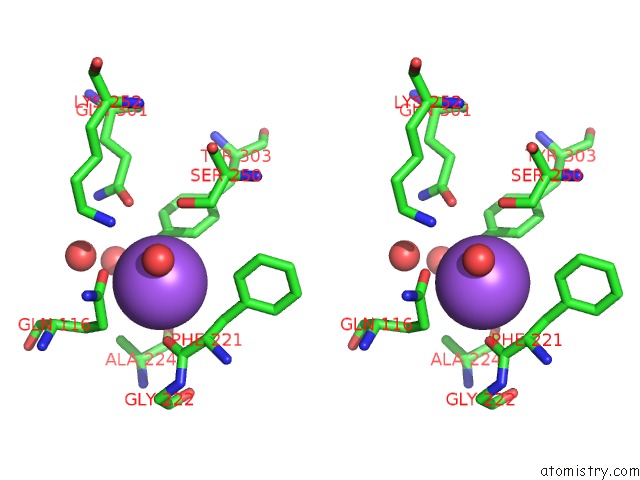
Sodium binding site 9 out
of 15 in the Crystal Structure of Listeriolysin O
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 9 of Crystal Structure of Listeriolysin O within 5.0Å range:
|
Sodium binding site 10 out of 15 in 4cdb
Go back to
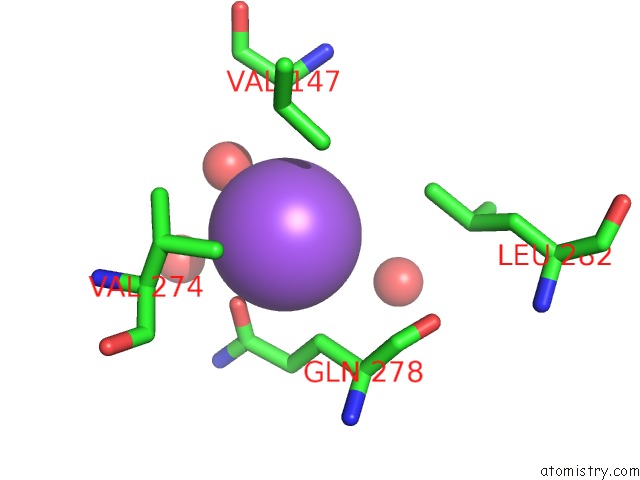
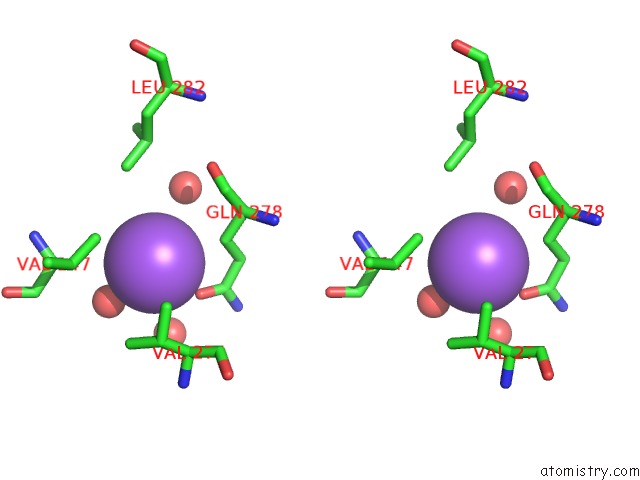
Sodium binding site 10 out
of 15 in the Crystal Structure of Listeriolysin O
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 10 of Crystal Structure of Listeriolysin O within 5.0Å range:
|
Reference:
S.Koester,
K.V.Pee,
M.Hudel,
M.Leustik,
D.Rhinow,
W.Kuehlbrandt,
T.Chakraborty,
O.Yildiz.
Crystal Structure of Listeriolysin O Reveals Molecular Details of Oligomerization and Pore Formation Nat.Commun. V. 5 3690 2014.
ISSN: ISSN 2041-1723
PubMed: 24751541
DOI: 10.1038/NCOMMS4690
Page generated: Sun Aug 17 18:46:45 2025
ISSN: ISSN 2041-1723
PubMed: 24751541
DOI: 10.1038/NCOMMS4690
Last articles
Na in 5IM9Na in 5IMD
Na in 5IMC
Na in 5IMA
Na in 5IIZ
Na in 5IIO
Na in 5ILF
Na in 5ILC
Na in 5IJS
Na in 5IJQ