Sodium »
PDB 3k9g-3la1 »
3kl0 »
Sodium in PDB 3kl0: Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis
Enzymatic activity of Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis
All present enzymatic activity of Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis:
3.2.1.136;
3.2.1.136;
Protein crystallography data
The structure of Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis, PDB code: 3kl0
was solved by
F.J.St John,
J.C.Hurlbert,
E.Pozharski,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 1.64 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 138.490, 195.796, 66.246, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.7 / 20.1 |
Sodium Binding Sites:
The binding sites of Sodium atom in the Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis
(pdb code 3kl0). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total 4 binding sites of Sodium where determined in the Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis, PDB code: 3kl0:
Jump to Sodium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Sodium where determined in the Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis, PDB code: 3kl0:
Jump to Sodium binding site number: 1; 2; 3; 4;
Sodium binding site 1 out of 4 in 3kl0
Go back to
Sodium binding site 1 out
of 4 in the Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis
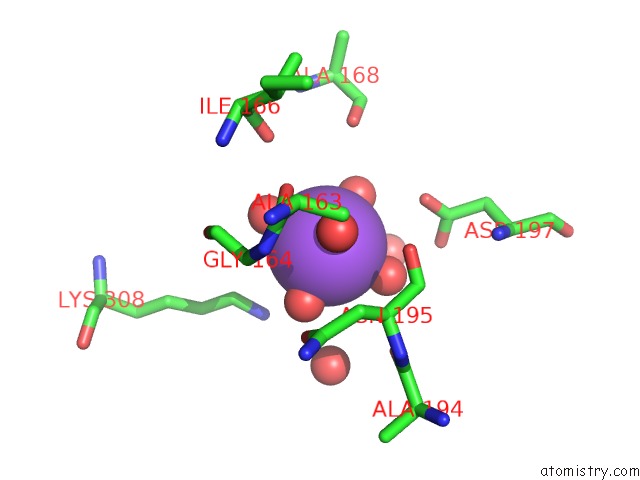
Mono view
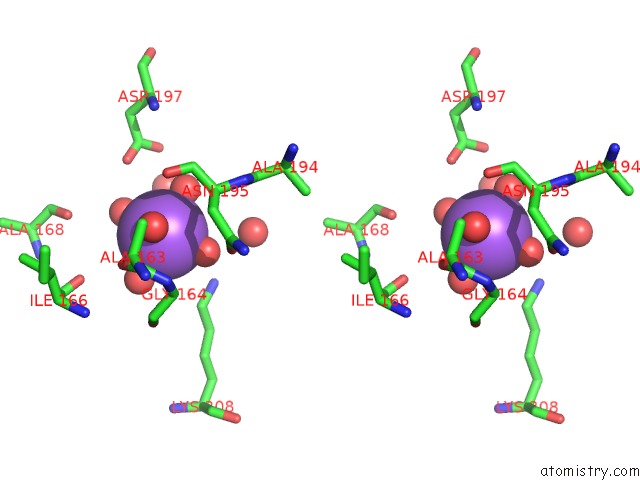
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis within 5.0Å range:
|
Sodium binding site 2 out of 4 in 3kl0
Go back to
Sodium binding site 2 out
of 4 in the Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis
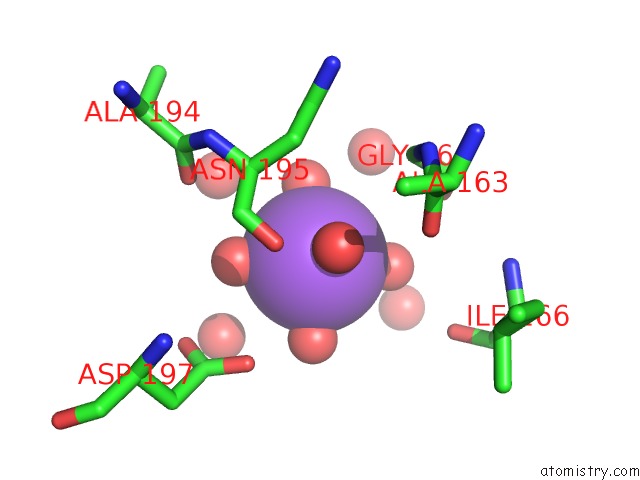
Mono view
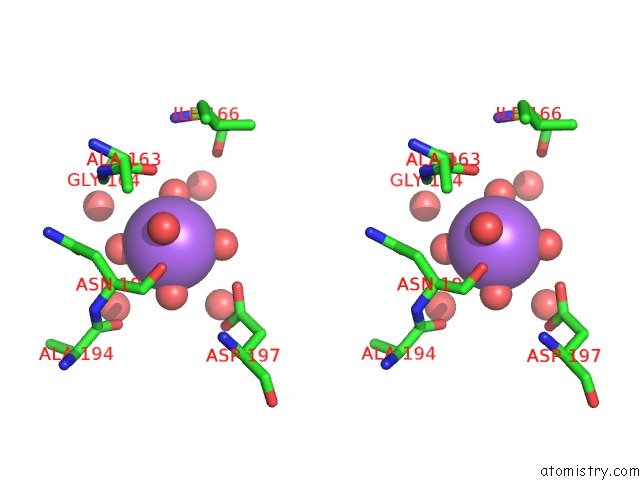
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 2 of Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis within 5.0Å range:
|
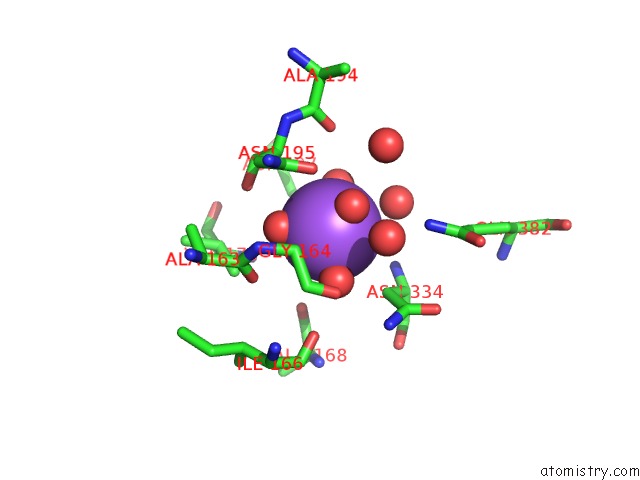
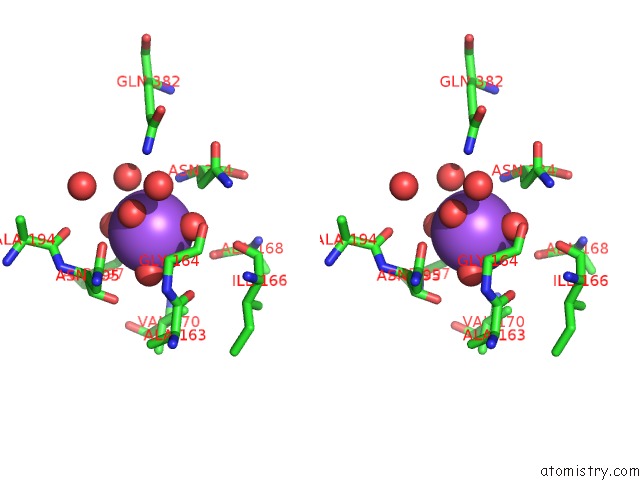
Sodium binding site 3 out of 4 in 3kl0
Go back to
Sodium binding site 3 out
of 4 in the Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 3 of Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis within 5.0Å range:
|
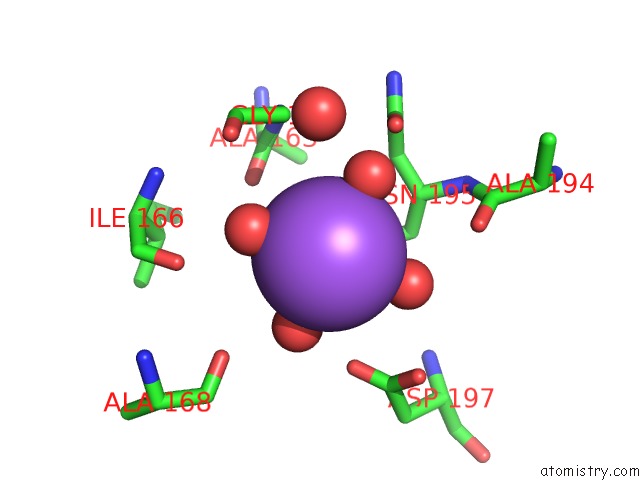
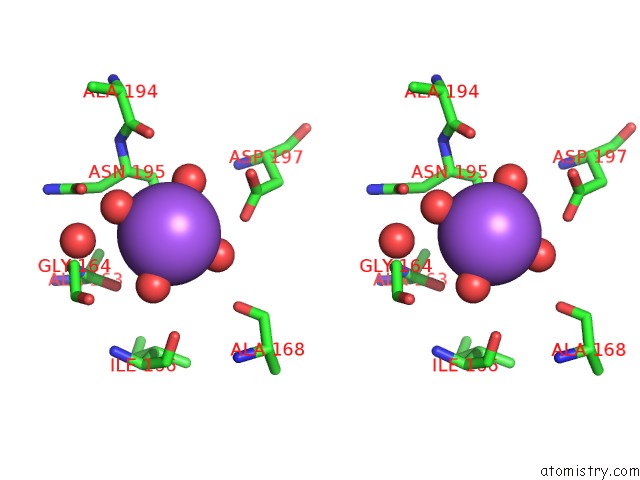
Sodium binding site 4 out of 4 in 3kl0
Go back to
Sodium binding site 4 out
of 4 in the Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 4 of Crystal Structure of the Glucuronoxylan Xylanohydrolase Xync From Bacillus Subtilis within 5.0Å range:
|
Reference:
F.J.St John,
J.C.Hurlbert,
J.D.Rice,
J.F.Preston,
E.Pozharski.
Ligand Bound Structures of A Glycosyl Hydrolase Family 30 Glucuronoxylan Xylanohydrolase. J.Mol.Biol. V. 407 92 2011.
ISSN: ISSN 0022-2836
PubMed: 21256135
DOI: 10.1016/J.JMB.2011.01.010
Page generated: Mon Oct 7 11:13:42 2024
ISSN: ISSN 0022-2836
PubMed: 21256135
DOI: 10.1016/J.JMB.2011.01.010
Last articles
Cl in 5G0BCl in 5FZO
Cl in 5FZL
Cl in 5FZK
Cl in 5FZM
Cl in 5FZI
Cl in 5FZH
Cl in 5FZG
Cl in 5FZF
Cl in 5FZE