Sodium »
PDB 3k9g-3la1 »
3kgq »
Sodium in PDB 3kgq: Carboxypeptidase A Liganded to An Organic Small-Molecule: Conformational Changes
Enzymatic activity of Carboxypeptidase A Liganded to An Organic Small-Molecule: Conformational Changes
All present enzymatic activity of Carboxypeptidase A Liganded to An Organic Small-Molecule: Conformational Changes:
3.4.17.1;
3.4.17.1;
Protein crystallography data
The structure of Carboxypeptidase A Liganded to An Organic Small-Molecule: Conformational Changes, PDB code: 3kgq
was solved by
D.Fernandez,
E.Boix,
I.Pallares,
F.X.Aviles,
J.Vendrell,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.24 / 1.70 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 40.604, 57.006, 60.604, 90.00, 102.04, 90.00 |
| R / Rfree (%) | 18 / 20.4 |
Other elements in 3kgq:
The structure of Carboxypeptidase A Liganded to An Organic Small-Molecule: Conformational Changes also contains other interesting chemical elements:
| Zinc | (Zn) | 1 atom |
Sodium Binding Sites:
The binding sites of Sodium atom in the Carboxypeptidase A Liganded to An Organic Small-Molecule: Conformational Changes
(pdb code 3kgq). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total only one binding site of Sodium was determined in the Carboxypeptidase A Liganded to An Organic Small-Molecule: Conformational Changes, PDB code: 3kgq:
In total only one binding site of Sodium was determined in the Carboxypeptidase A Liganded to An Organic Small-Molecule: Conformational Changes, PDB code: 3kgq:
Sodium binding site 1 out of 1 in 3kgq
Go back to
Sodium binding site 1 out
of 1 in the Carboxypeptidase A Liganded to An Organic Small-Molecule: Conformational Changes
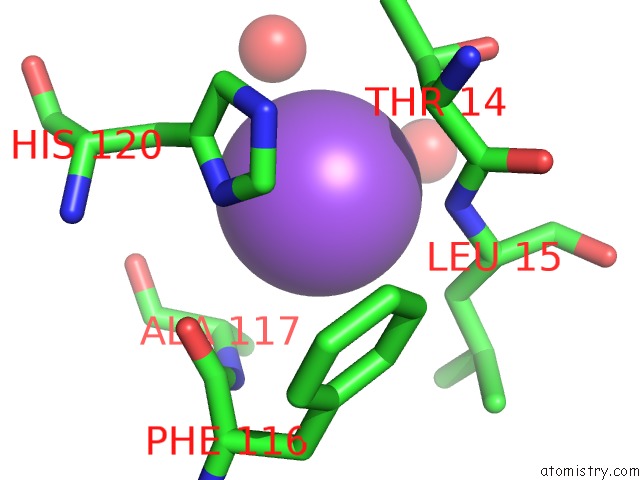
Mono view
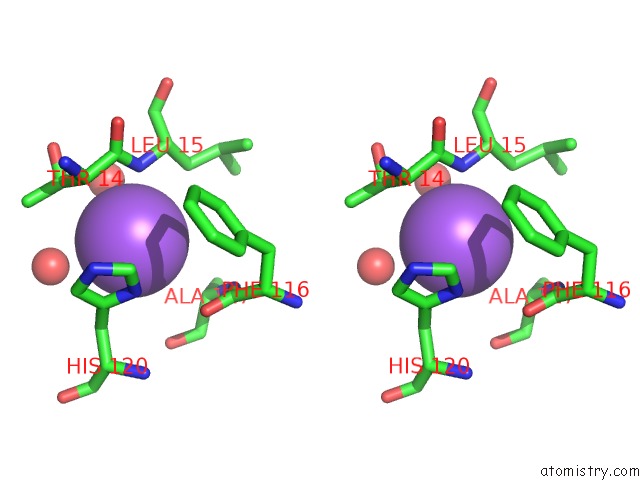
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Carboxypeptidase A Liganded to An Organic Small-Molecule: Conformational Changes within 5.0Å range:
|
Reference:
D.Fernandez,
E.Boix,
I.Pallares,
F.X.Aviles,
J.Vendrell.
Structural and Functional Analysis of the Complex Between Citrate and the Zinc Peptidase Carboxypeptidase A Enzyme Res V.2011 28676 2011.
ISSN: ISSN 2090-0406
PubMed: 21804935
DOI: 10.4061/2011/128676
Page generated: Mon Oct 7 11:13:12 2024
ISSN: ISSN 2090-0406
PubMed: 21804935
DOI: 10.4061/2011/128676
Last articles
Cl in 7ZIKCl in 7ZIZ
Cl in 7ZI0
Cl in 7ZIC
Cl in 7ZH8
Cl in 7ZGO
Cl in 7ZHF
Cl in 7ZH9
Cl in 7ZGA
Cl in 7ZGL