Sodium »
PDB 3iwf-3k93 »
3k6n »
Sodium in PDB 3k6n: Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain
Protein crystallography data
The structure of Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain, PDB code: 3k6n
was solved by
Y.Xu,
H.G.Shin,
S.Szep,
Z.Lu,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.00 / 2.00 |
| Space group | P 4 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 77.455, 77.455, 86.723, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 25.4 / 26.6 |
Sodium Binding Sites:
The binding sites of Sodium atom in the Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain
(pdb code 3k6n). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total 5 binding sites of Sodium where determined in the Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain, PDB code: 3k6n:
Jump to Sodium binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Sodium where determined in the Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain, PDB code: 3k6n:
Jump to Sodium binding site number: 1; 2; 3; 4; 5;
Sodium binding site 1 out of 5 in 3k6n
Go back to
Sodium binding site 1 out
of 5 in the Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain within 5.0Å range:
|
Sodium binding site 2 out of 5 in 3k6n
Go back to
Sodium binding site 2 out
of 5 in the Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain
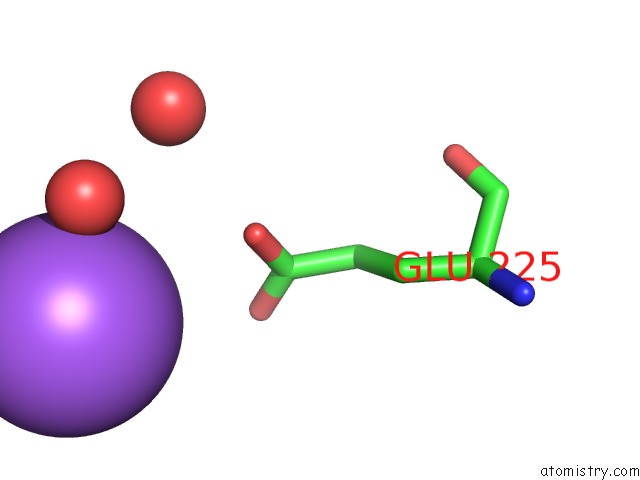
Mono view
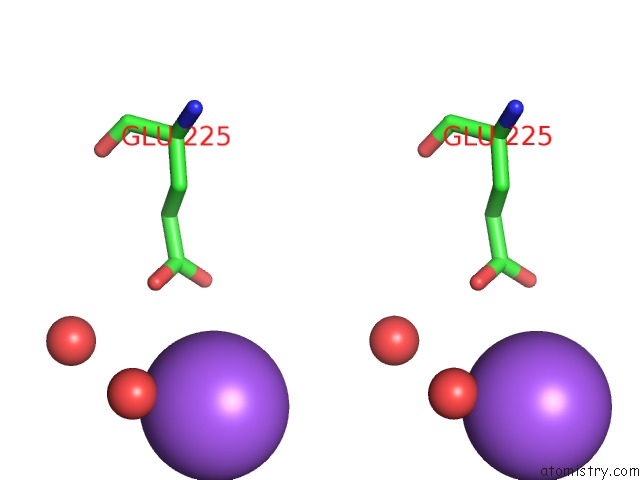
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 2 of Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain within 5.0Å range:
|
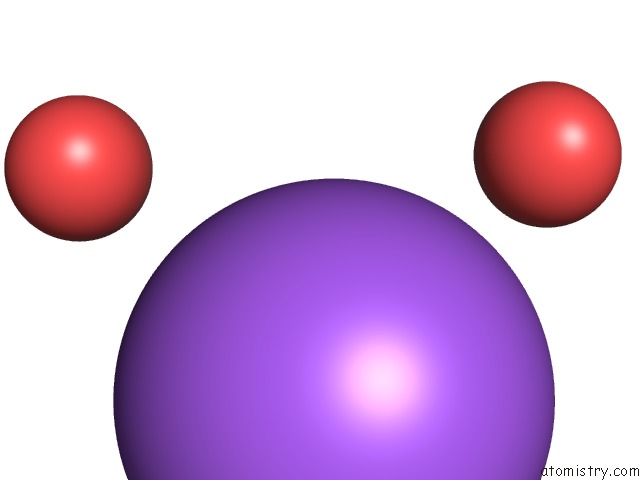
Sodium binding site 3 out of 5 in 3k6n
Go back to
Sodium binding site 3 out
of 5 in the Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain
Mono view
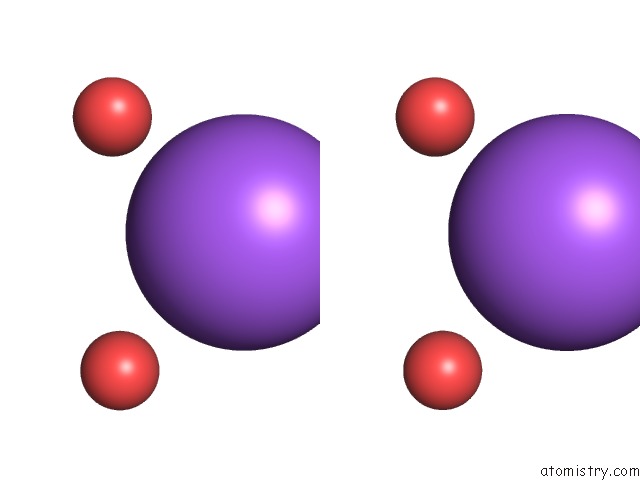
Stereo pair view

Mono view
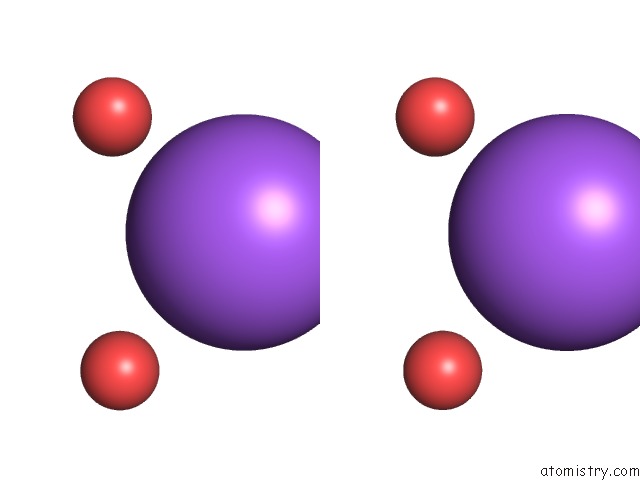
Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 3 of Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain within 5.0Å range:
|
Sodium binding site 4 out of 5 in 3k6n
Go back to
Sodium binding site 4 out
of 5 in the Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain
Mono view
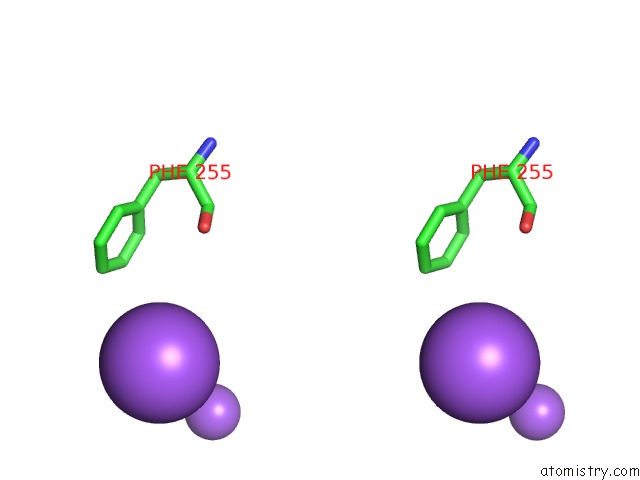
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 4 of Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain within 5.0Å range:
|
Sodium binding site 5 out of 5 in 3k6n
Go back to
Sodium binding site 5 out
of 5 in the Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain
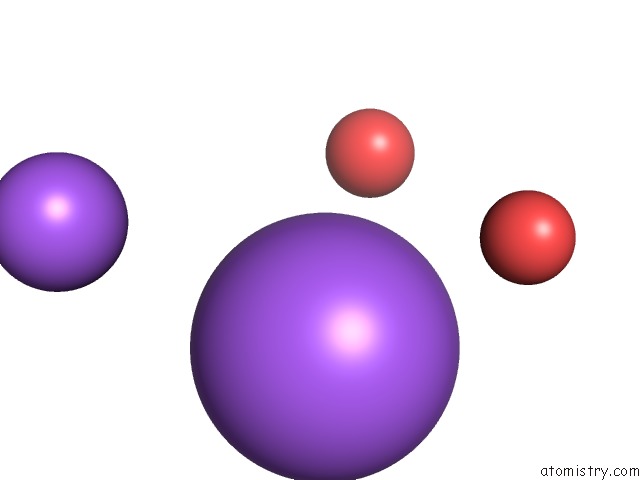
Mono view
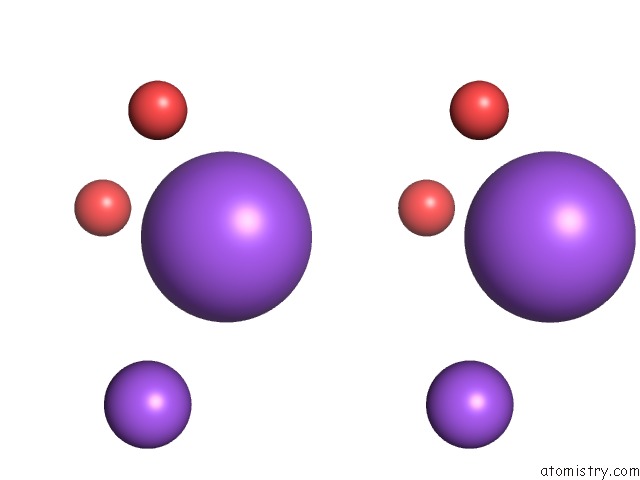
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 5 of Crystal Structure of the S225E Mutant KIR3.1 Cytoplasmic Pore Domain within 5.0Å range:
|
Reference:
Y.Xu,
H.G.Shin,
S.Szep,
Z.Lu.
Physical Determinants of Strong Voltage Sensitivity of K(+) Channel Block. Nat.Struct.Mol.Biol. V. 16 1252 2009.
ISSN: ISSN 1545-9993
PubMed: 19915587
DOI: 10.1038/NSMB.1717
Page generated: Mon Oct 7 11:11:03 2024
ISSN: ISSN 1545-9993
PubMed: 19915587
DOI: 10.1038/NSMB.1717
Last articles
Cl in 5IA3Cl in 5IAE
Cl in 5IBN
Cl in 5IBC
Cl in 5IB5
Cl in 5IAB
Cl in 5IA0
Cl in 5I7A
Cl in 5IA1
Cl in 5I9Y