Sodium »
PDB 1hnf-1jay »
1i2s »
Sodium in PDB 1i2s: Beta-Lactamase From Bacillus Licheniformis BS3
Enzymatic activity of Beta-Lactamase From Bacillus Licheniformis BS3
All present enzymatic activity of Beta-Lactamase From Bacillus Licheniformis BS3:
3.5.2.6;
3.5.2.6;
Protein crystallography data
The structure of Beta-Lactamase From Bacillus Licheniformis BS3, PDB code: 1i2s
was solved by
E.Fonze,
M.Vanhove,
G.Dive,
E.Sauvage,
J.M.Frere,
P.Charlier,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 8.00 / 1.70 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 47.362, 106.817, 63.901, 90.00, 94.43, 90.00 |
| R / Rfree (%) | 19.7 / 23.7 |
Sodium Binding Sites:
The binding sites of Sodium atom in the Beta-Lactamase From Bacillus Licheniformis BS3
(pdb code 1i2s). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total 2 binding sites of Sodium where determined in the Beta-Lactamase From Bacillus Licheniformis BS3, PDB code: 1i2s:
Jump to Sodium binding site number: 1; 2;
In total 2 binding sites of Sodium where determined in the Beta-Lactamase From Bacillus Licheniformis BS3, PDB code: 1i2s:
Jump to Sodium binding site number: 1; 2;
Sodium binding site 1 out of 2 in 1i2s
Go back to
Sodium binding site 1 out
of 2 in the Beta-Lactamase From Bacillus Licheniformis BS3
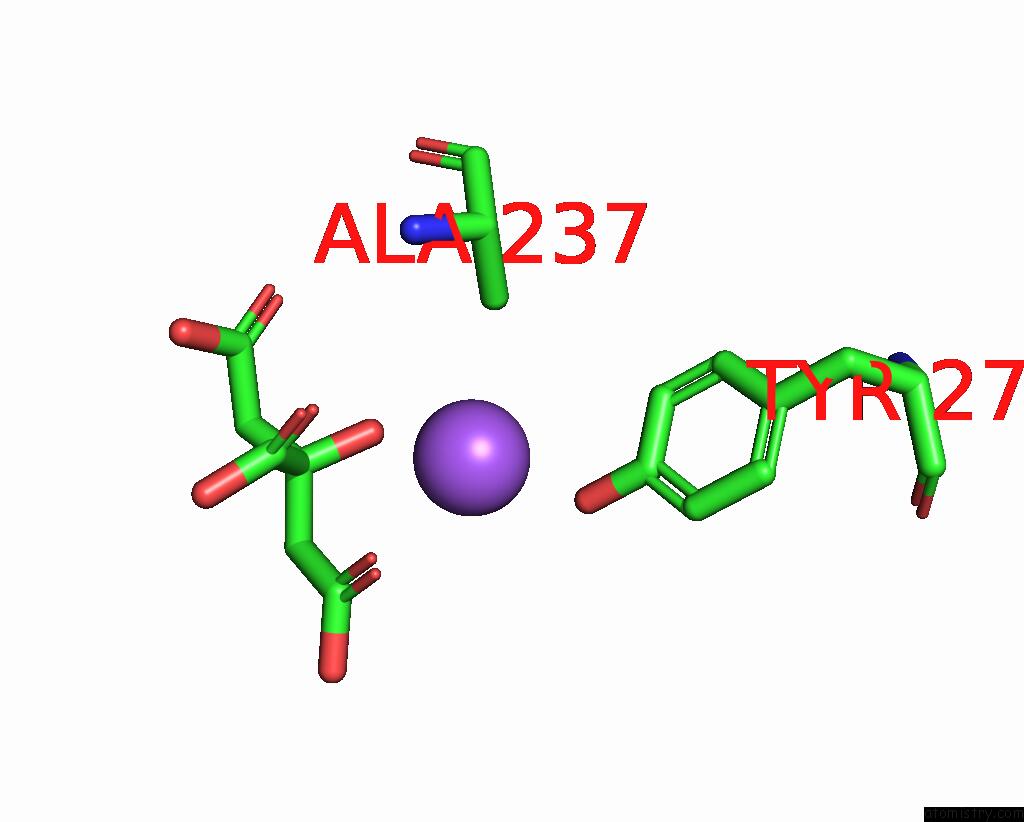
Mono view
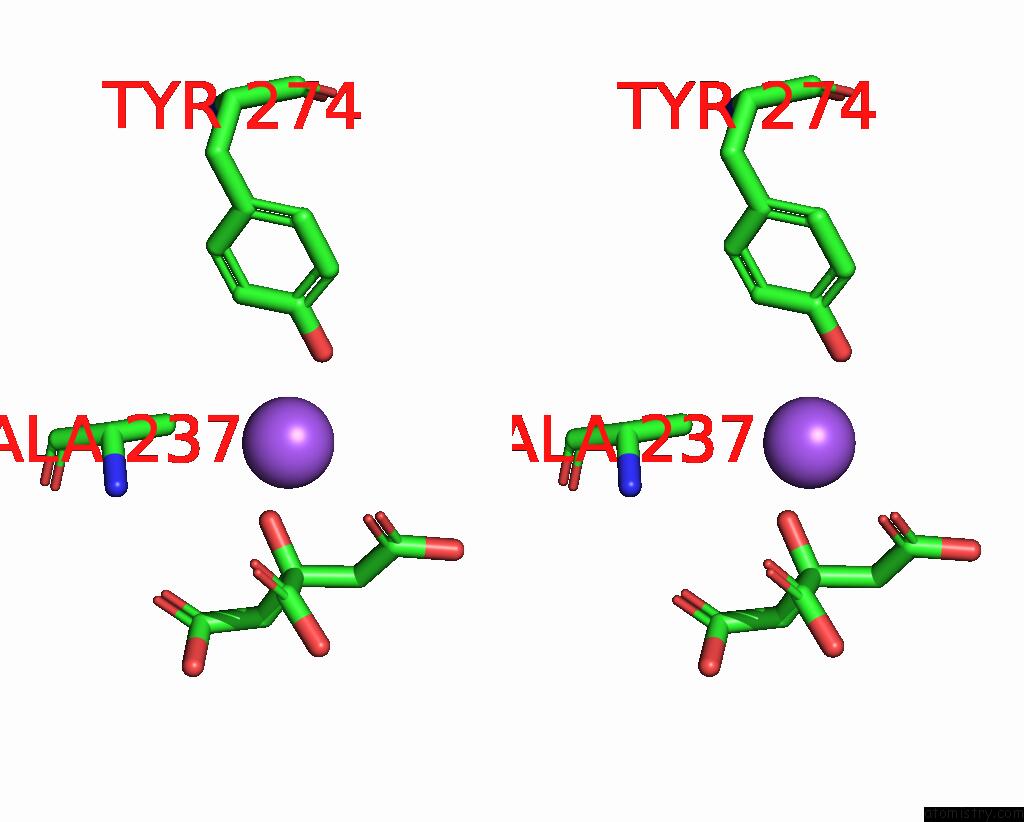
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Beta-Lactamase From Bacillus Licheniformis BS3 within 5.0Å range:
|
Sodium binding site 2 out of 2 in 1i2s
Go back to
Sodium binding site 2 out
of 2 in the Beta-Lactamase From Bacillus Licheniformis BS3
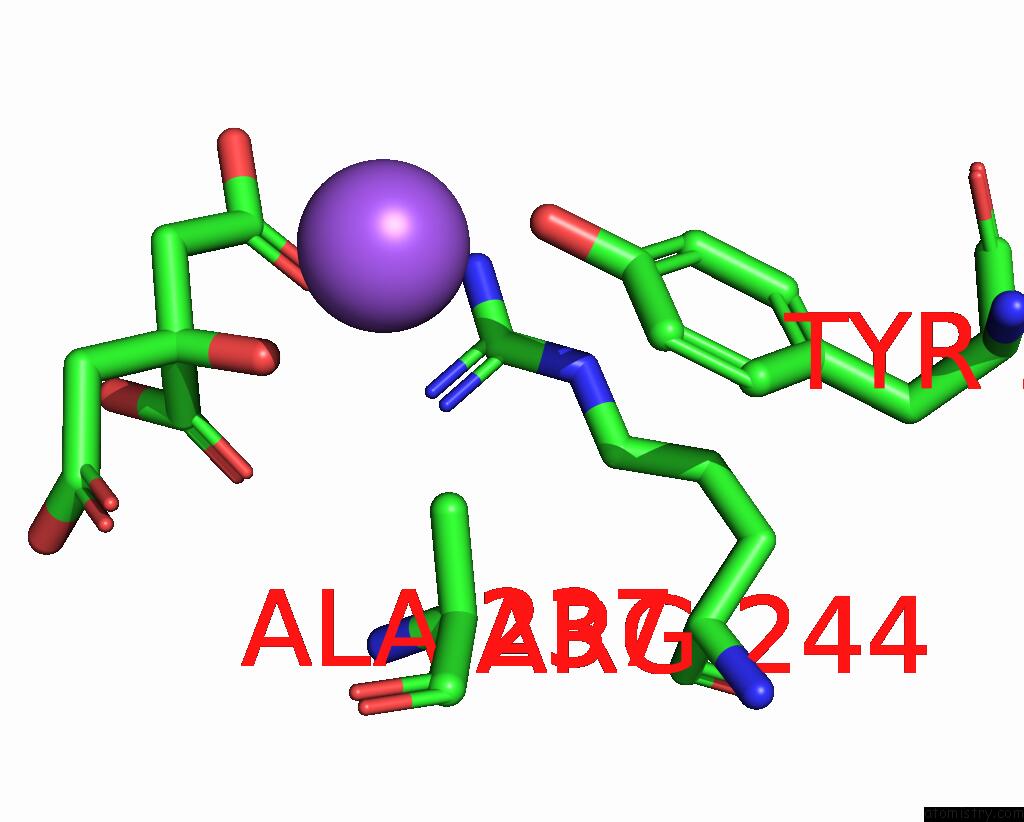
Mono view
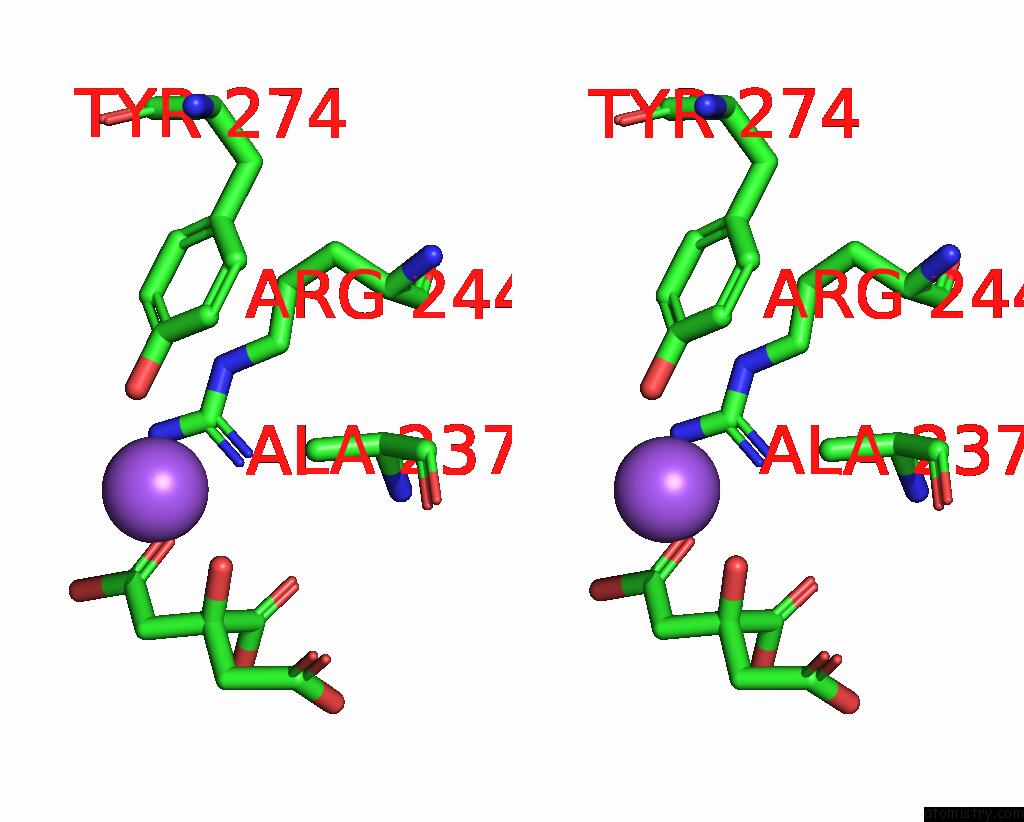
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 2 of Beta-Lactamase From Bacillus Licheniformis BS3 within 5.0Å range:
|
Reference:
E.Fonze,
M.Vanhove,
G.Dive,
E.Sauvage,
J.M.Frere,
P.Charlier.
Crystal Structures of the Bacillus Licheniformis BS3 Class A Beta-Lactamase and of the Acyl-Enzyme Adduct Formed with Cefoxitin Biochemistry V. 41 1877 2002.
ISSN: ISSN 0006-2960
PubMed: 11827533
DOI: 10.1021/BI015789K
Page generated: Sun Aug 17 05:16:30 2025
ISSN: ISSN 0006-2960
PubMed: 11827533
DOI: 10.1021/BI015789K
Last articles
Na in 3DDKNa in 3DFH
Na in 3DEB
Na in 3DAV
Na in 3DDR
Na in 3DBO
Na in 3DC7
Na in 3D97
Na in 3DA9
Na in 3D9R