Sodium »
PDB 5l6q-5lq6 »
5lc1 »
Sodium in PDB 5lc1: L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound.
Enzymatic activity of L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound.
All present enzymatic activity of L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound.:
1.1.1.103;
1.1.1.103;
Protein crystallography data
The structure of L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound., PDB code: 5lc1
was solved by
P.T.Erskine,
E.Adjogatse,
S.P.Wood,
J.B.Cooper,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 95.50 / 2.10 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 132.040, 276.490, 55.740, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 14.7 / 20.8 |
Sodium Binding Sites:
The binding sites of Sodium atom in the L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound.
(pdb code 5lc1). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total 6 binding sites of Sodium where determined in the L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound., PDB code: 5lc1:
Jump to Sodium binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Sodium where determined in the L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound., PDB code: 5lc1:
Jump to Sodium binding site number: 1; 2; 3; 4; 5; 6;
Sodium binding site 1 out of 6 in 5lc1
Go back to
Sodium binding site 1 out
of 6 in the L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound.
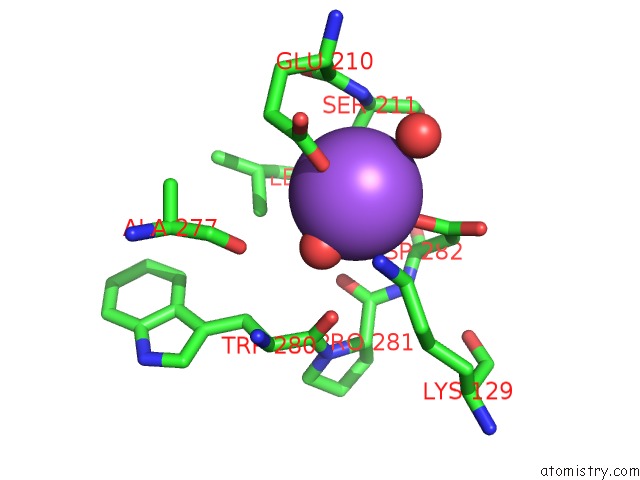
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound. within 5.0Å range:
|
Sodium binding site 2 out of 6 in 5lc1
Go back to
Sodium binding site 2 out
of 6 in the L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound.
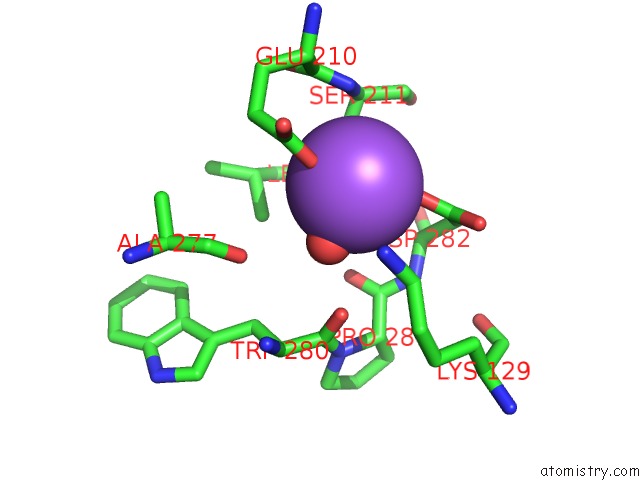
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 2 of L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound. within 5.0Å range:
|
Sodium binding site 3 out of 6 in 5lc1
Go back to
Sodium binding site 3 out
of 6 in the L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound.
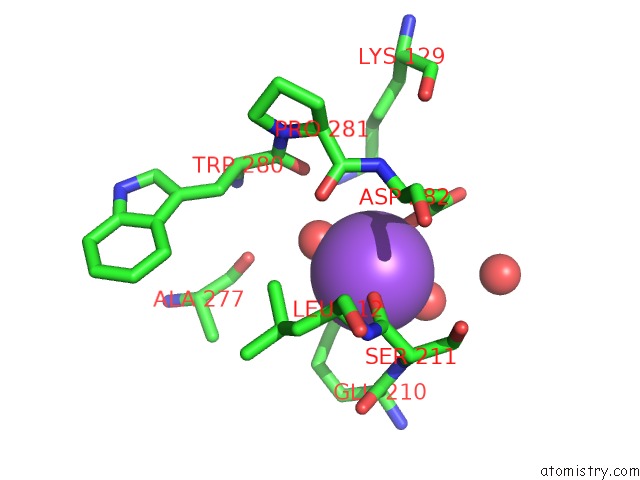
Mono view
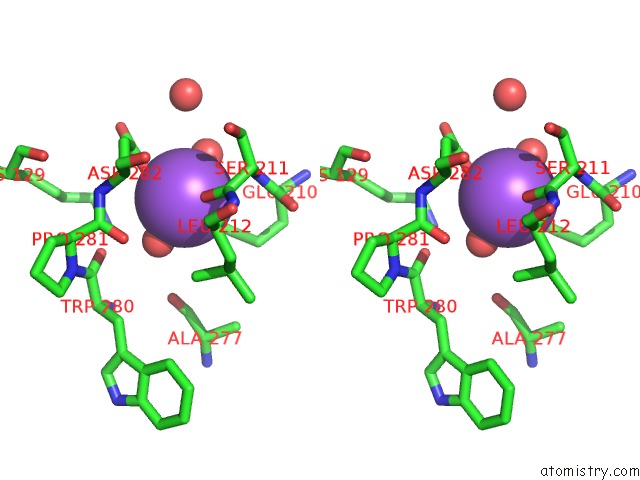
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 3 of L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound. within 5.0Å range:
|
Sodium binding site 4 out of 6 in 5lc1
Go back to
Sodium binding site 4 out
of 6 in the L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound.
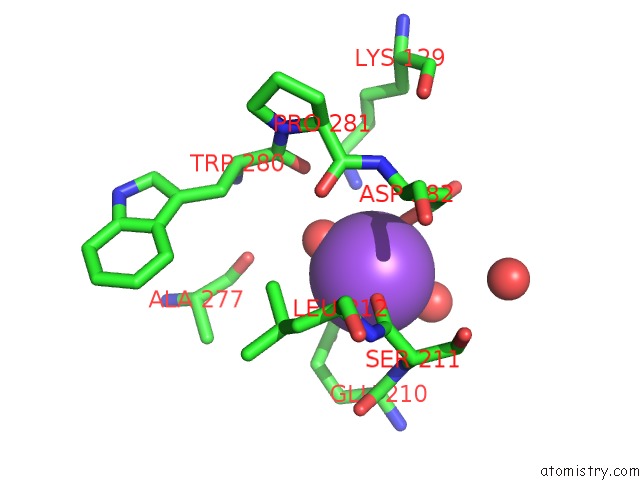
Mono view
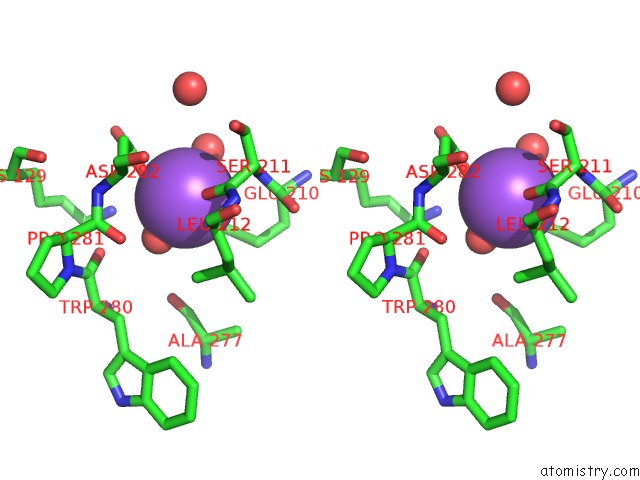
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 4 of L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound. within 5.0Å range:
|
Sodium binding site 5 out of 6 in 5lc1
Go back to
Sodium binding site 5 out
of 6 in the L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound.
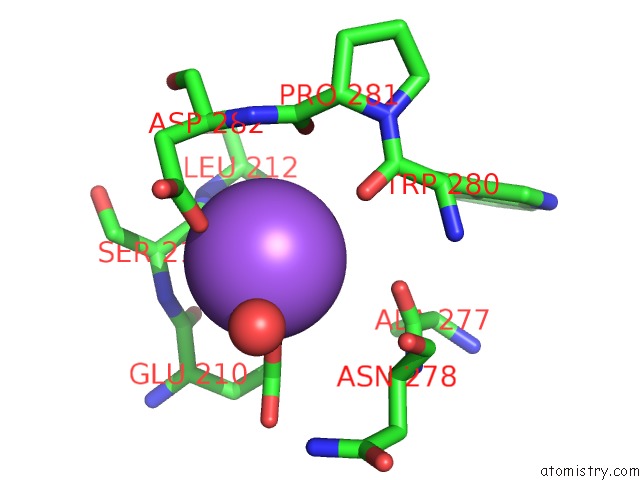
Mono view
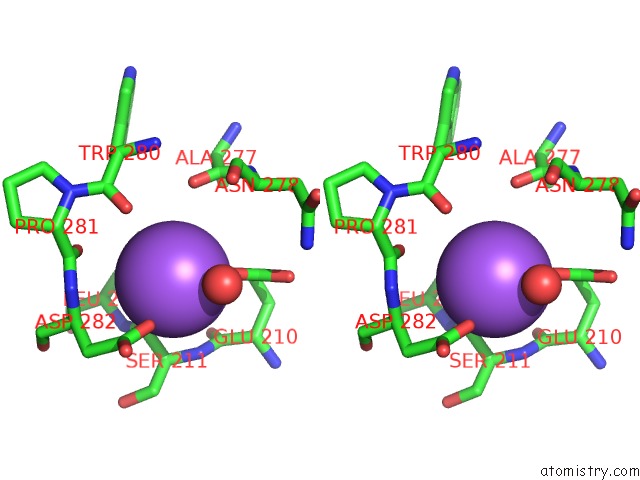
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 5 of L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound. within 5.0Å range:
|
Sodium binding site 6 out of 6 in 5lc1
Go back to
Sodium binding site 6 out
of 6 in the L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound.
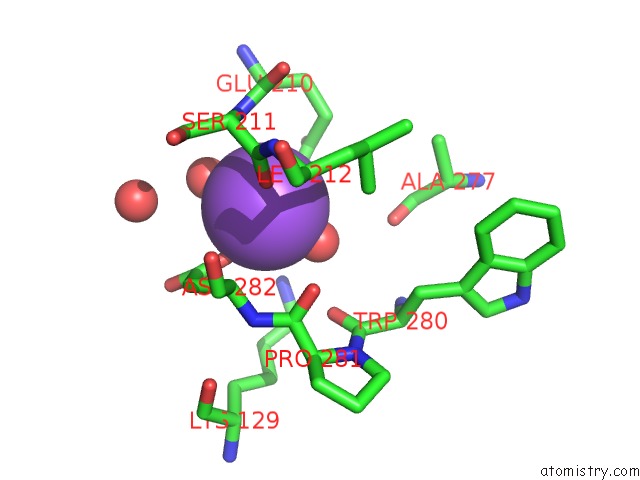
Mono view
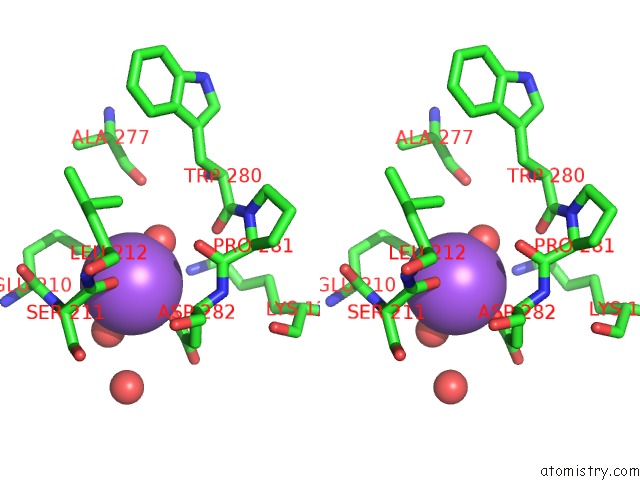
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 6 of L-Threonine Dehydrogenase From Trypanosoma Brucei with Nad and the Inhibitor Pyruvate Bound. within 5.0Å range:
|
Reference:
E.Adjogatse,
P.Erskine,
S.A.Wells,
J.M.Kelly,
J.D.Wilden,
A.W.E.Chan,
D.Selwood,
A.Coker,
S.Wood,
J.B.Cooper.
Structure and Function of L-Threonine-3-Dehydrogenase From the Parasitic Protozoan Trypanosoma Brucei Revealed By X-Ray Crystallography and Geometric Simulations. Acta Crystallogr D Struct V. 74 861 2018BIOL.
ISSN: ISSN 2059-7983
PubMed: 30198897
DOI: 10.1107/S2059798318009208
Page generated: Mon Oct 7 22:19:56 2024
ISSN: ISSN 2059-7983
PubMed: 30198897
DOI: 10.1107/S2059798318009208
Last articles
I in 6DFNI in 6DMN
I in 6DEO
I in 6D64
I in 6DEN
I in 6D0Y
I in 6CVL
I in 6CST
I in 6CG4
I in 6BZ5