Sodium »
PDB 6p9k-6puk »
6pbr »
Sodium in PDB 6pbr: Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group
Enzymatic activity of Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group
All present enzymatic activity of Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group:
2.3.1.61;
2.3.1.61;
Protein crystallography data
The structure of Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group, PDB code: 6pbr
was solved by
B.Andi,
A.S.Soares,
W.Shi,
M.R.Fuchs,
S.Mcsweeney,
Q.Liu,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.36 / 3.00 |
| Space group | I 4 |
| Cell size a, b, c (Å), α, β, γ (°) | 128.598, 128.598, 249.733, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 23 / 27.2 |
Sodium Binding Sites:
The binding sites of Sodium atom in the Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group
(pdb code 6pbr). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total 6 binding sites of Sodium where determined in the Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group, PDB code: 6pbr:
Jump to Sodium binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Sodium where determined in the Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group, PDB code: 6pbr:
Jump to Sodium binding site number: 1; 2; 3; 4; 5; 6;
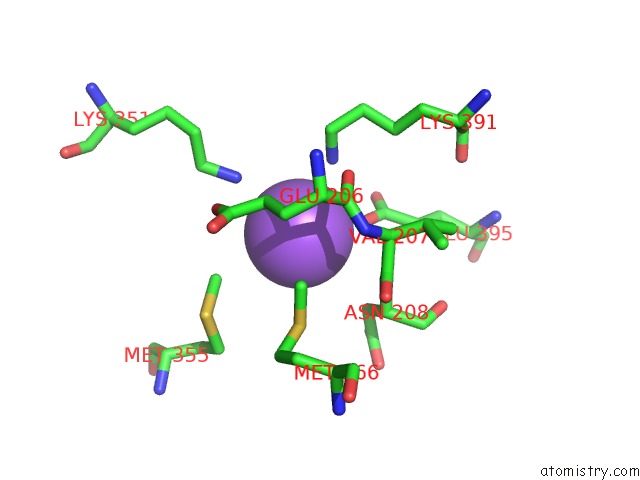
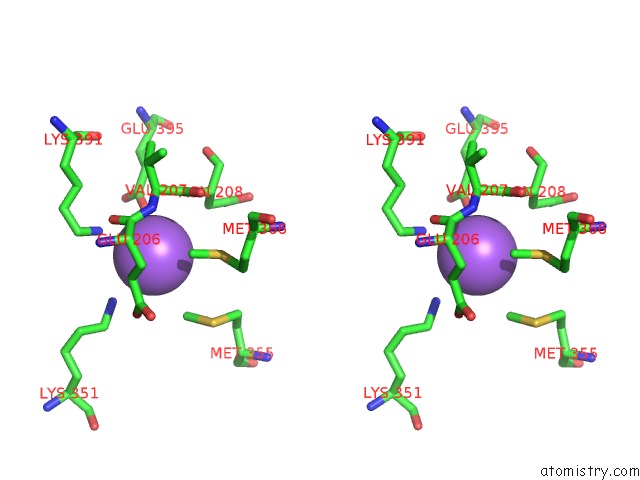
Sodium binding site 1 out of 6 in 6pbr
Go back to
Sodium binding site 1 out
of 6 in the Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group within 5.0Å range:
|
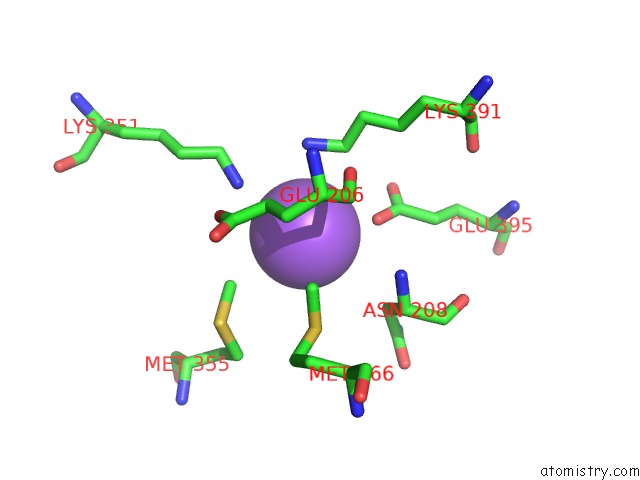
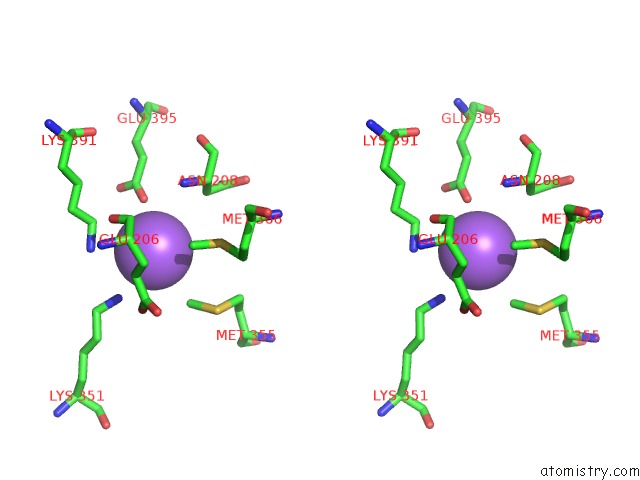
Sodium binding site 2 out of 6 in 6pbr
Go back to
Sodium binding site 2 out
of 6 in the Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 2 of Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group within 5.0Å range:
|
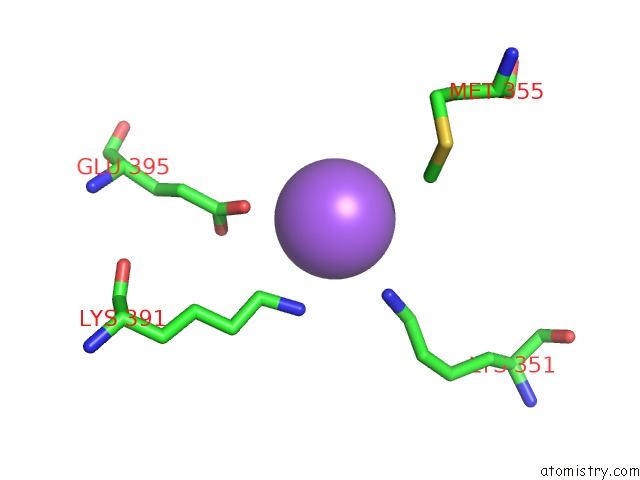
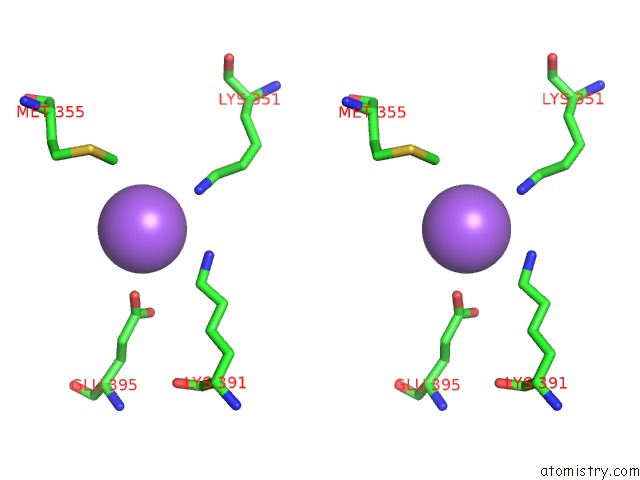
Sodium binding site 3 out of 6 in 6pbr
Go back to
Sodium binding site 3 out
of 6 in the Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 3 of Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group within 5.0Å range:
|
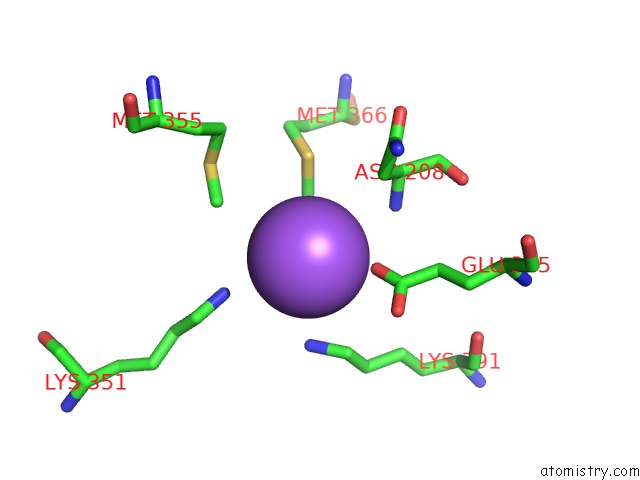
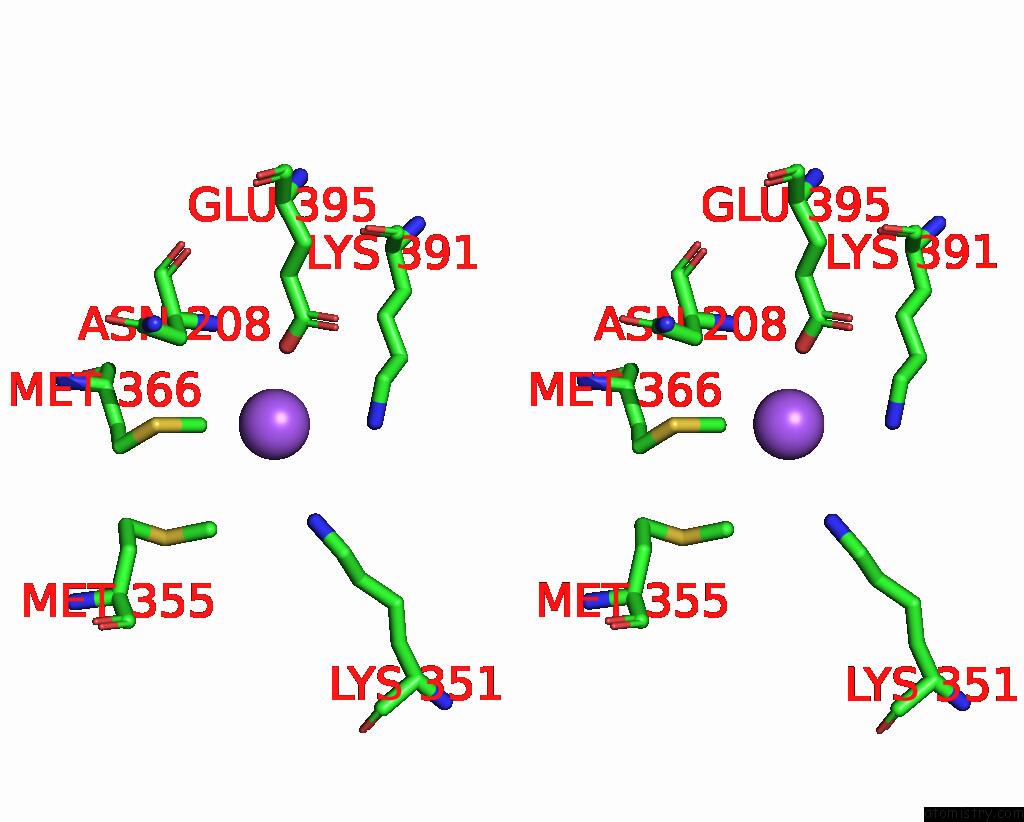
Sodium binding site 4 out of 6 in 6pbr
Go back to
Sodium binding site 4 out
of 6 in the Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 4 of Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group within 5.0Å range:
|
Sodium binding site 5 out of 6 in 6pbr
Go back to
Sodium binding site 5 out
of 6 in the Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group
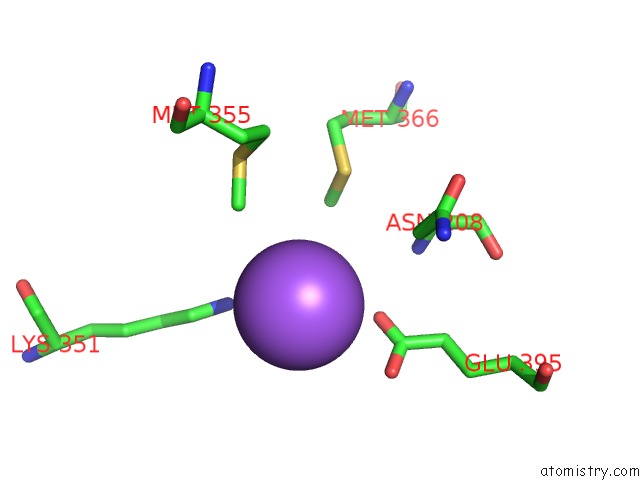
Mono view
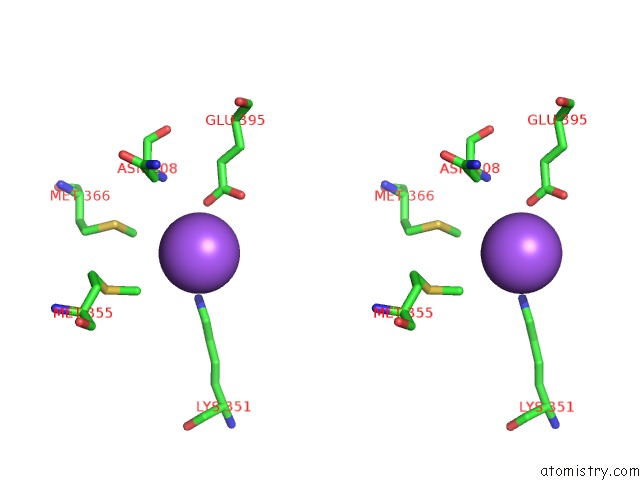
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 5 of Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group within 5.0Å range:
|
Sodium binding site 6 out of 6 in 6pbr
Go back to
Sodium binding site 6 out
of 6 in the Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group
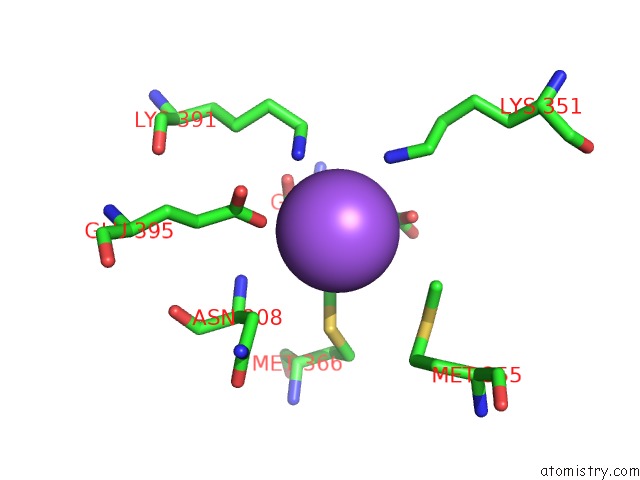
Mono view
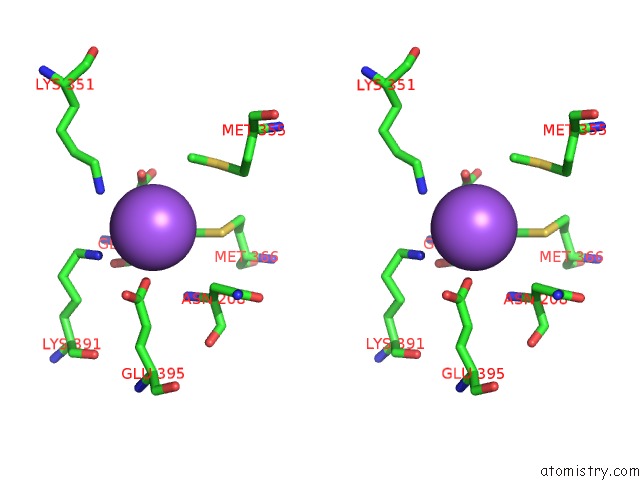
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 6 of Catalytic Domain of E.Coli Dihydrolipoamide Succinyltransferase in I4 Space Group within 5.0Å range:
|
Reference:
B.Andi,
A.S.Soares,
W.Shi,
M.R.Fuchs,
S.Mcsweeney,
Q.Liu.
Structure of the Dihydrolipoamide Succinyltransferase Catalytic Domain From Escherichia Coli in A Novel Crystal Form: A Tale of A Common Protein Crystallization Contaminant. Acta Crystallogr.,Sect.F V. 75 616 2019.
ISSN: ESSN 2053-230X
PubMed: 31475929
DOI: 10.1107/S2053230X19011488
Page generated: Tue Oct 8 12:42:13 2024
ISSN: ESSN 2053-230X
PubMed: 31475929
DOI: 10.1107/S2053230X19011488
Last articles
F in 7QMAF in 7QM6
F in 7QM9
F in 7QM8
F in 7QM7
F in 7QM5
F in 7QM4
F in 7QLT
F in 7QIT
F in 7QM3