Sodium »
PDB 5zxe-6ah7 »
6aa9 »
Sodium in PDB 6aa9: T166A Mutant of D-Serine Deaminase From Salmonella Typhimurium
Enzymatic activity of T166A Mutant of D-Serine Deaminase From Salmonella Typhimurium
All present enzymatic activity of T166A Mutant of D-Serine Deaminase From Salmonella Typhimurium:
4.3.1.18;
4.3.1.18;
Protein crystallography data
The structure of T166A Mutant of D-Serine Deaminase From Salmonella Typhimurium, PDB code: 6aa9
was solved by
G.Deka,
S.R.Bharath,
H.S.Shavithri,
M.R.N.Murthy,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 53.20 / 2.70 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 108.310, 46.140, 99.280, 90.00, 100.80, 90.00 |
| R / Rfree (%) | 28.9 / 34.9 |
Sodium Binding Sites:
The binding sites of Sodium atom in the T166A Mutant of D-Serine Deaminase From Salmonella Typhimurium
(pdb code 6aa9). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total only one binding site of Sodium was determined in the T166A Mutant of D-Serine Deaminase From Salmonella Typhimurium, PDB code: 6aa9:
In total only one binding site of Sodium was determined in the T166A Mutant of D-Serine Deaminase From Salmonella Typhimurium, PDB code: 6aa9:
Sodium binding site 1 out of 1 in 6aa9
Go back to
Sodium binding site 1 out
of 1 in the T166A Mutant of D-Serine Deaminase From Salmonella Typhimurium
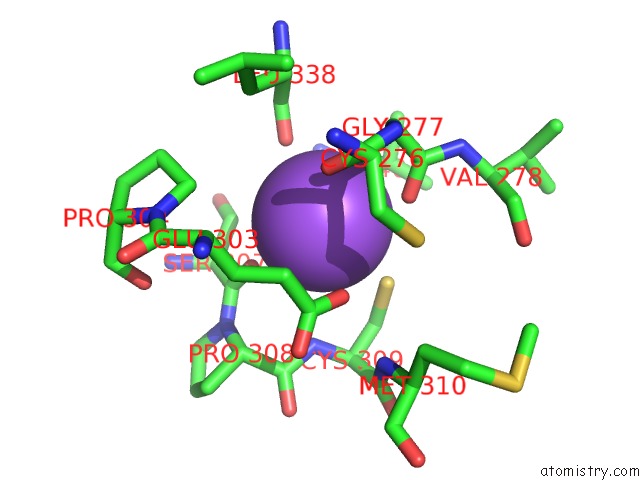
Mono view
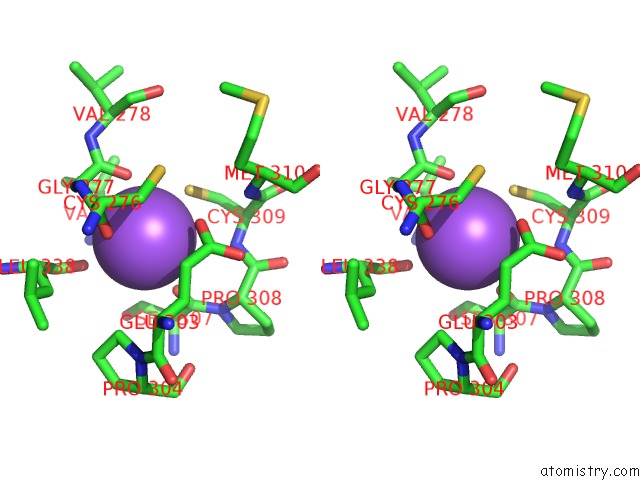
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of T166A Mutant of D-Serine Deaminase From Salmonella Typhimurium within 5.0Å range:
|
Reference:
G.Deka,
S.R.Bharath,
H.S.Shavithri,
M.R.N.Murthy.
Structural Studies on the Decameric S. Typhimurium Arginine Decarboxylase (Adc): Pyridoxal 5'-Phosphate Binding Induces Conformational Changes Biochem. Biophys. Res. V. 490 1362 2017COMMUN..
ISSN: ESSN 1090-2104
PubMed: 28694189
DOI: 10.1016/J.BBRC.2017.07.032
Page generated: Tue Oct 8 01:46:45 2024
ISSN: ESSN 1090-2104
PubMed: 28694189
DOI: 10.1016/J.BBRC.2017.07.032
Last articles
Ca in 5MNKCa in 5MNH
Ca in 5MNG
Ca in 5MNF
Ca in 5MNE
Ca in 5MNC
Ca in 5MNB
Ca in 5MKG
Ca in 5MN1
Ca in 5MNA