Sodium »
PDB 5dyn-5emg »
5edf »
Sodium in PDB 5edf: Crystal Structure of the Selenomethionine-Substituted Iron-Regulated Protein Frpd From Neisseria Meningitidis
Protein crystallography data
The structure of Crystal Structure of the Selenomethionine-Substituted Iron-Regulated Protein Frpd From Neisseria Meningitidis, PDB code: 5edf
was solved by
E.Sviridova,
L.Bumba,
P.Rezacova,
P.Sebo,
I.Kuta Smatanova,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 25.20 / 1.40 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 38.293, 38.829, 165.670, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.8 / 19.3 |
Sodium Binding Sites:
The binding sites of Sodium atom in the Crystal Structure of the Selenomethionine-Substituted Iron-Regulated Protein Frpd From Neisseria Meningitidis
(pdb code 5edf). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total only one binding site of Sodium was determined in the Crystal Structure of the Selenomethionine-Substituted Iron-Regulated Protein Frpd From Neisseria Meningitidis, PDB code: 5edf:
In total only one binding site of Sodium was determined in the Crystal Structure of the Selenomethionine-Substituted Iron-Regulated Protein Frpd From Neisseria Meningitidis, PDB code: 5edf:
Sodium binding site 1 out of 1 in 5edf
Go back to
Sodium binding site 1 out
of 1 in the Crystal Structure of the Selenomethionine-Substituted Iron-Regulated Protein Frpd From Neisseria Meningitidis
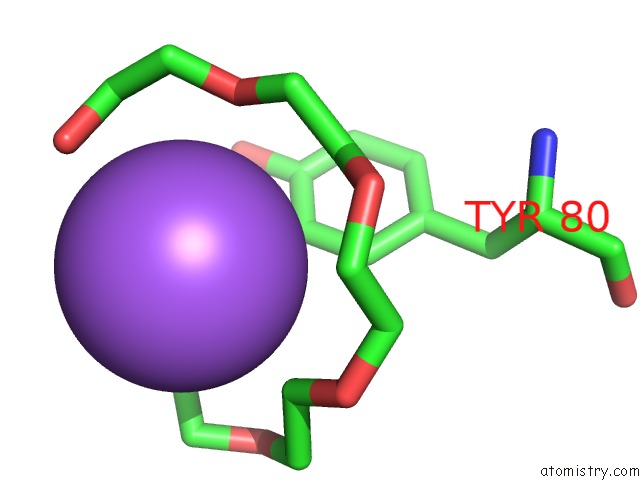
Mono view
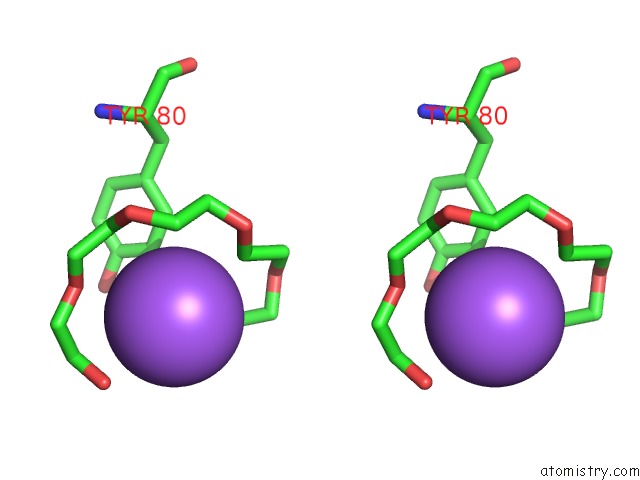
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Crystal Structure of the Selenomethionine-Substituted Iron-Regulated Protein Frpd From Neisseria Meningitidis within 5.0Å range:
|
Reference:
E.Sviridova,
P.Rezacova,
A.Bondar,
V.Veverka,
P.Novak,
G.Schenk,
D.I.Svergun,
I.Kuta Smatanova,
L.Bumba.
Structural Basis of the Interaction Between the Putative Adhesion-Involved and Iron-Regulated Frpd and Frpc Proteins of Neisseria Meningitidis. Sci Rep V. 7 40408 2017.
ISSN: ESSN 2045-2322
PubMed: 28084396
DOI: 10.1038/SREP40408
Page generated: Mon Oct 7 20:45:16 2024
ISSN: ESSN 2045-2322
PubMed: 28084396
DOI: 10.1038/SREP40408
Last articles
Cl in 5F92Cl in 5F8P
Cl in 5F6Y
Cl in 5F6X
Cl in 5F7R
Cl in 5F6Q
Cl in 5F4W
Cl in 5F62
Cl in 5F63
Cl in 5F4Q