Sodium »
PDB 2woi-2x2v »
2x0r »
Sodium in PDB 2x0r: R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform)
Enzymatic activity of R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform)
All present enzymatic activity of R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform):
1.1.1.37;
1.1.1.37;
Protein crystallography data
The structure of R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform), PDB code: 2x0r
was solved by
A.Irimia,
C.Ebel,
F.M.D.Vellieux,
S.B.Richard,
L.W.Cosenza,
G.Zaccai,
D.Madern,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 44.50 / 2.92 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 115.360, 125.910, 125.840, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.4 / 28.6 |
Other elements in 2x0r:
The structure of R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform) also contains other interesting chemical elements:
| Chlorine | (Cl) | 4 atoms |
Sodium Binding Sites:
The binding sites of Sodium atom in the R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform)
(pdb code 2x0r). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total 5 binding sites of Sodium where determined in the R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform), PDB code: 2x0r:
Jump to Sodium binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Sodium where determined in the R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform), PDB code: 2x0r:
Jump to Sodium binding site number: 1; 2; 3; 4; 5;
Sodium binding site 1 out of 5 in 2x0r
Go back to
Sodium binding site 1 out
of 5 in the R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform)
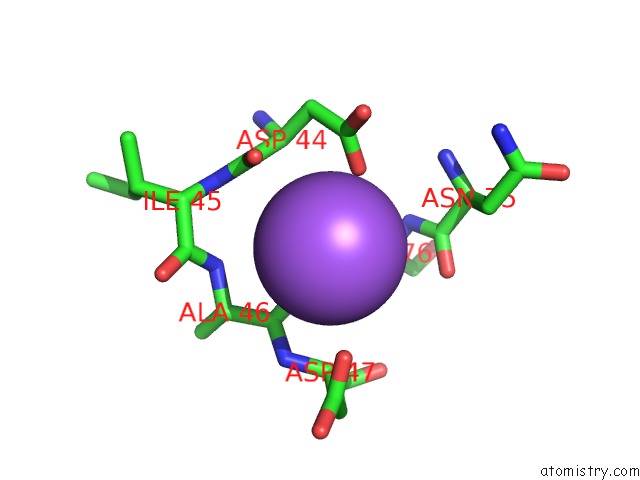
Mono view
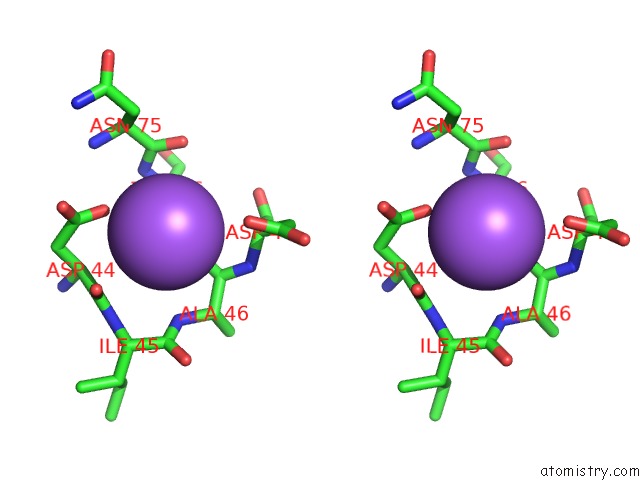
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform) within 5.0Å range:
|
Sodium binding site 2 out of 5 in 2x0r
Go back to
Sodium binding site 2 out
of 5 in the R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform)
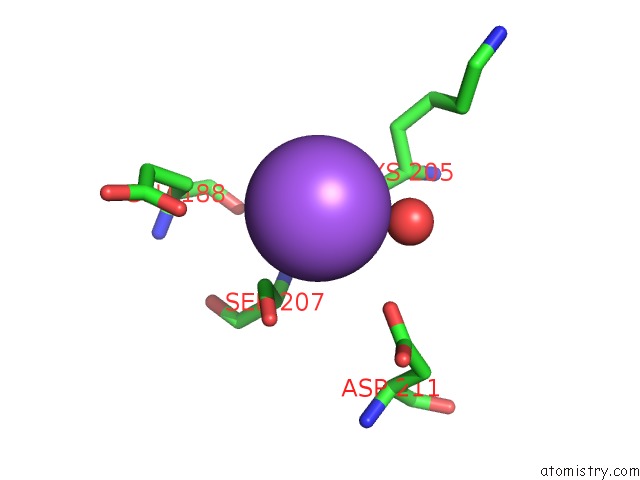
Mono view
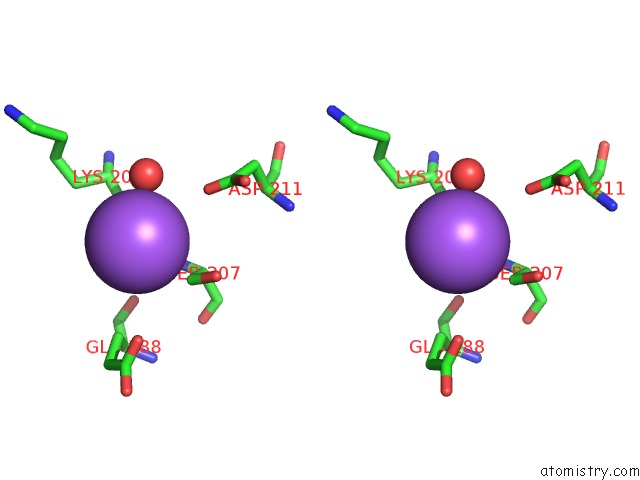
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 2 of R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform) within 5.0Å range:
|
Sodium binding site 3 out of 5 in 2x0r
Go back to
Sodium binding site 3 out
of 5 in the R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform)
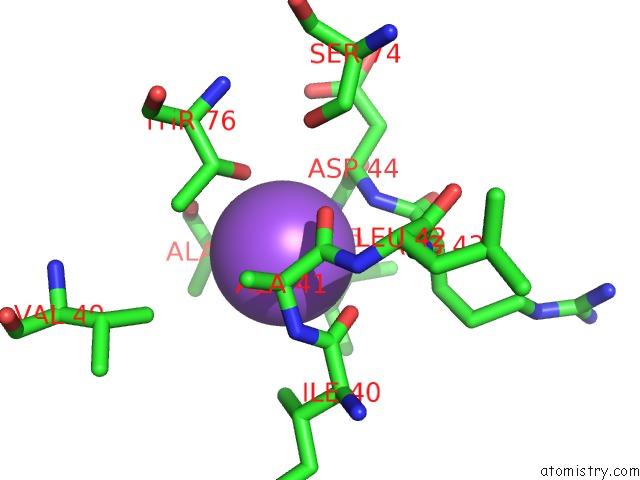
Mono view
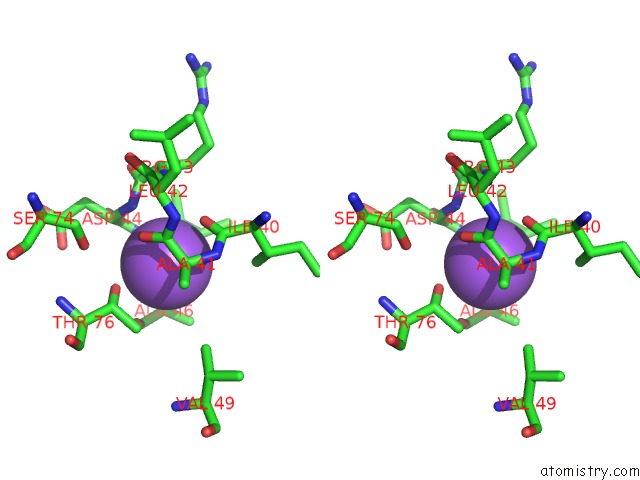
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 3 of R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform) within 5.0Å range:
|
Sodium binding site 4 out of 5 in 2x0r
Go back to
Sodium binding site 4 out
of 5 in the R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform)
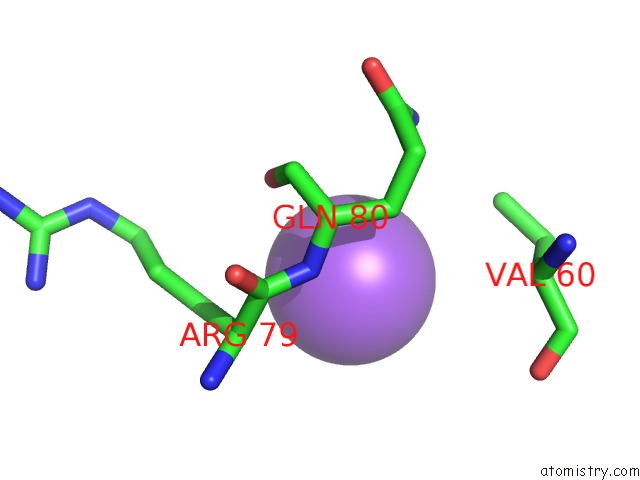
Mono view
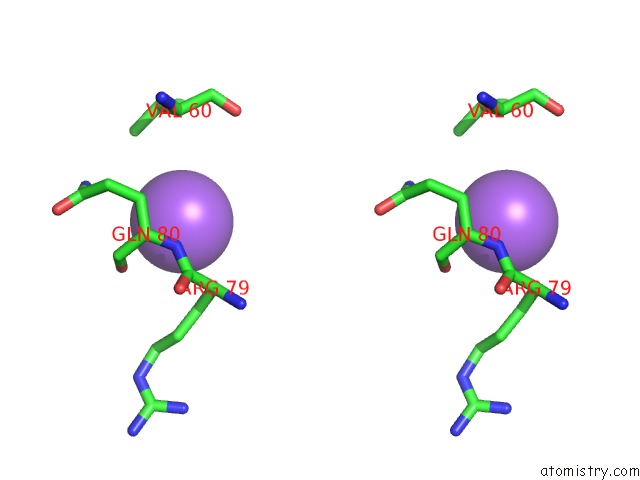
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 4 of R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform) within 5.0Å range:
|
Sodium binding site 5 out of 5 in 2x0r
Go back to
Sodium binding site 5 out
of 5 in the R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform)
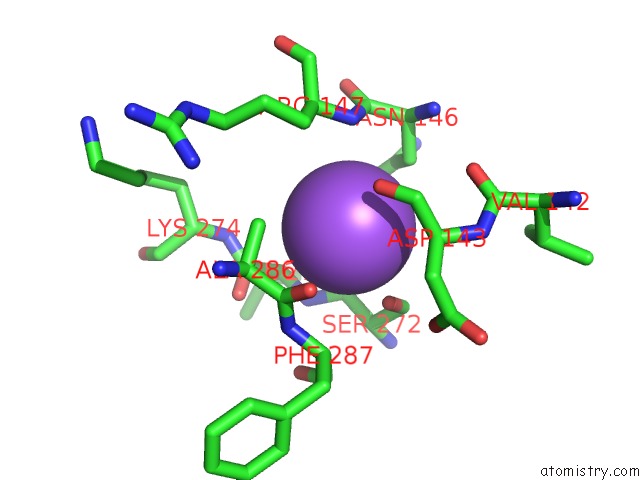
Mono view
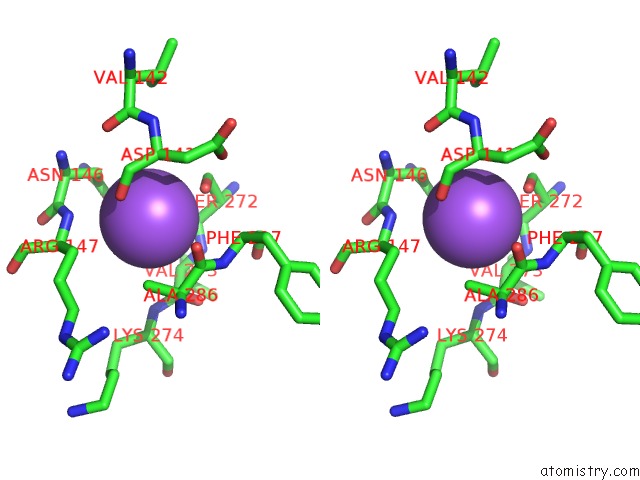
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 5 of R207S, R292S Mutant of Malate Dehydrogenase From the Halophilic Archeon Haloarcula Marismortui (Holoform) within 5.0Å range:
|
Reference:
A.Irimia,
C.Ebel,
D.Madern,
S.B.Richard,
L.W.Cosenza,
G.Zaccai,
F.M.D.Vellieux.
The Oligomeric States of Haloarcula Marismortui Malate Dehydrogenase Are Modulated By Solvent Components As Shown By Crystallographic and Biochemical Studies J.Mol.Biol. V. 326 859 2003.
ISSN: ISSN 0022-2836
PubMed: 12581646
DOI: 10.1016/S0022-2836(02)01450-X
Page generated: Mon Oct 7 05:02:43 2024
ISSN: ISSN 0022-2836
PubMed: 12581646
DOI: 10.1016/S0022-2836(02)01450-X
Last articles
Cl in 5FGOCl in 5FGP
Cl in 5FGK
Cl in 5FGG
Cl in 5FGF
Cl in 5FGE
Cl in 5FGD
Cl in 5FGA
Cl in 5FG7
Cl in 5FF3