Sodium »
PDB 2ply-2qd6 »
2q92 »
Sodium in PDB 2q92: E. Coli Methionine Aminopeptidase Mn-Form with Inhibitor B23
Enzymatic activity of E. Coli Methionine Aminopeptidase Mn-Form with Inhibitor B23
All present enzymatic activity of E. Coli Methionine Aminopeptidase Mn-Form with Inhibitor B23:
3.4.11.18;
3.4.11.18;
Protein crystallography data
The structure of E. Coli Methionine Aminopeptidase Mn-Form with Inhibitor B23, PDB code: 2q92
was solved by
Q.-Z.Ye,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 26.40 / 1.90 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 38.137, 60.984, 50.693, 90.00, 104.93, 90.00 |
| R / Rfree (%) | 20.3 / 23.8 |
Other elements in 2q92:
The structure of E. Coli Methionine Aminopeptidase Mn-Form with Inhibitor B23 also contains other interesting chemical elements:
| Manganese | (Mn) | 2 atoms |
Sodium Binding Sites:
The binding sites of Sodium atom in the E. Coli Methionine Aminopeptidase Mn-Form with Inhibitor B23
(pdb code 2q92). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total only one binding site of Sodium was determined in the E. Coli Methionine Aminopeptidase Mn-Form with Inhibitor B23, PDB code: 2q92:
In total only one binding site of Sodium was determined in the E. Coli Methionine Aminopeptidase Mn-Form with Inhibitor B23, PDB code: 2q92:
Sodium binding site 1 out of 1 in 2q92
Go back to
Sodium binding site 1 out
of 1 in the E. Coli Methionine Aminopeptidase Mn-Form with Inhibitor B23
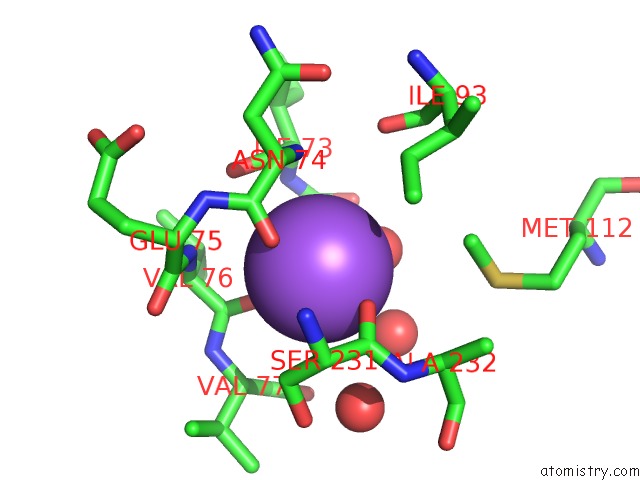
Mono view
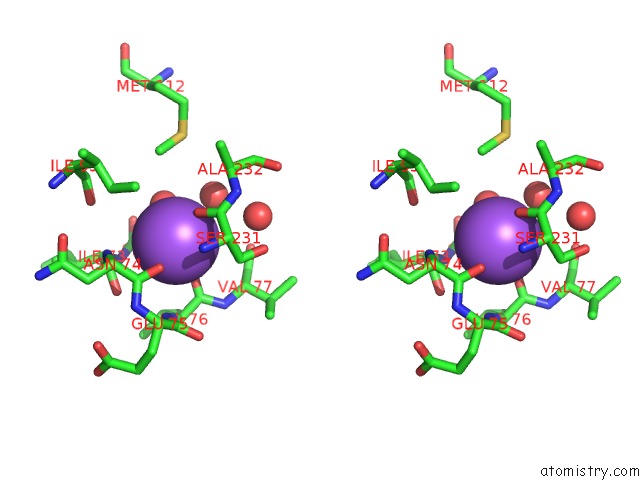
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of E. Coli Methionine Aminopeptidase Mn-Form with Inhibitor B23 within 5.0Å range:
|
Reference:
Z.Q.Ma,
S.X.Xie,
Q.Q.Huang,
F.J.Nan,
T.D.Hurley,
Q.Z.Ye.
Structural Analysis of Inhibition of E. Coli Methionine Aminopeptidase: Implication of Loop Flexibility in Selective Inhibition of Bacterial Enzymes. Bmc Struct.Biol. V. 7 84 2007.
ISSN: ESSN 1472-6807
PubMed: 18093325
DOI: 10.1186/1472-6807-7-84
Page generated: Mon Oct 7 03:48:00 2024
ISSN: ESSN 1472-6807
PubMed: 18093325
DOI: 10.1186/1472-6807-7-84
Last articles
Cl in 5GWBCl in 5GWA
Cl in 5GVP
Cl in 5GVN
Cl in 5GVM
Cl in 5GTY
Cl in 5GVL
Cl in 5GVK
Cl in 5GUS
Cl in 5GUW