Sodium »
PDB 2czs-2e4r »
2d3n »
Sodium in PDB 2d3n: Crystal Structure of Maltohexaose-Producing Amylase From Bacillus Sp.707 Complexed with Maltohexaose
Enzymatic activity of Crystal Structure of Maltohexaose-Producing Amylase From Bacillus Sp.707 Complexed with Maltohexaose
All present enzymatic activity of Crystal Structure of Maltohexaose-Producing Amylase From Bacillus Sp.707 Complexed with Maltohexaose:
3.2.1.98;
3.2.1.98;
Protein crystallography data
The structure of Crystal Structure of Maltohexaose-Producing Amylase From Bacillus Sp.707 Complexed with Maltohexaose, PDB code: 2d3n
was solved by
R.Kanai,
K.Haga,
T.Akiba,
K.Yamane,
K.Harata,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 6.00 / 1.90 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 47.630, 82.690, 126.970, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 15.5 / 18.4 |
Other elements in 2d3n:
The structure of Crystal Structure of Maltohexaose-Producing Amylase From Bacillus Sp.707 Complexed with Maltohexaose also contains other interesting chemical elements:
| Calcium | (Ca) | 3 atoms |
Sodium Binding Sites:
The binding sites of Sodium atom in the Crystal Structure of Maltohexaose-Producing Amylase From Bacillus Sp.707 Complexed with Maltohexaose
(pdb code 2d3n). This binding sites where shown within
5.0 Angstroms radius around Sodium atom.
In total only one binding site of Sodium was determined in the Crystal Structure of Maltohexaose-Producing Amylase From Bacillus Sp.707 Complexed with Maltohexaose, PDB code: 2d3n:
In total only one binding site of Sodium was determined in the Crystal Structure of Maltohexaose-Producing Amylase From Bacillus Sp.707 Complexed with Maltohexaose, PDB code: 2d3n:
Sodium binding site 1 out of 1 in 2d3n
Go back to
Sodium binding site 1 out
of 1 in the Crystal Structure of Maltohexaose-Producing Amylase From Bacillus Sp.707 Complexed with Maltohexaose
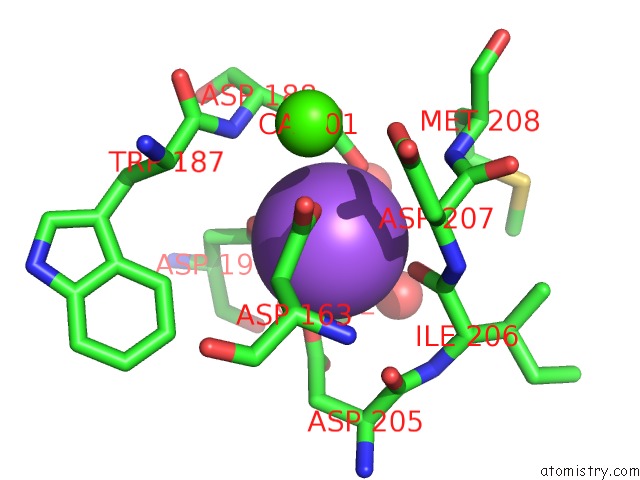
Mono view
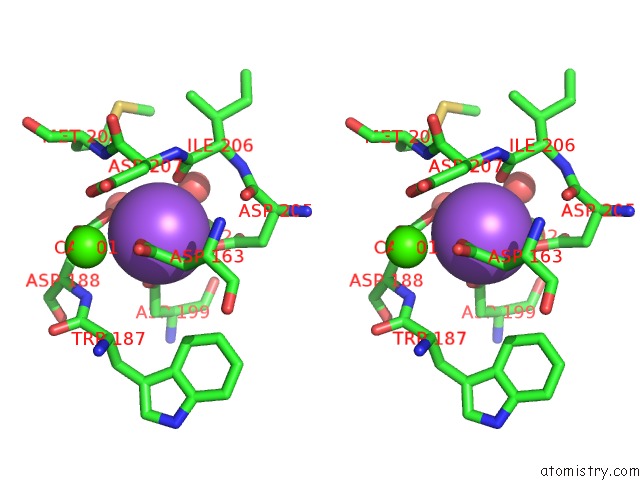
Stereo pair view

Mono view

Stereo pair view
A full contact list of Sodium with other atoms in the Na binding
site number 1 of Crystal Structure of Maltohexaose-Producing Amylase From Bacillus Sp.707 Complexed with Maltohexaose within 5.0Å range:
|
Reference:
R.Kanai,
K.Haga,
T.Akiba,
K.Yamane,
K.Harata.
Role of TRP140 at Subsite -6 on the Maltohexaose Production of Maltohexaose-Producing Amylase From Alkalophilic Bacillus Sp.707 Protein Sci. V. 15 468 2006.
ISSN: ISSN 0961-8368
PubMed: 16452622
DOI: 10.1110/PS.051877006
Page generated: Mon Oct 7 02:11:03 2024
ISSN: ISSN 0961-8368
PubMed: 16452622
DOI: 10.1110/PS.051877006
Last articles
Cl in 5EE3Cl in 5EE7
Cl in 5EDI
Cl in 5EDG
Cl in 5EDE
Cl in 5EDT
Cl in 5EDQ
Cl in 5ED7
Cl in 5EDC
Cl in 5EAH